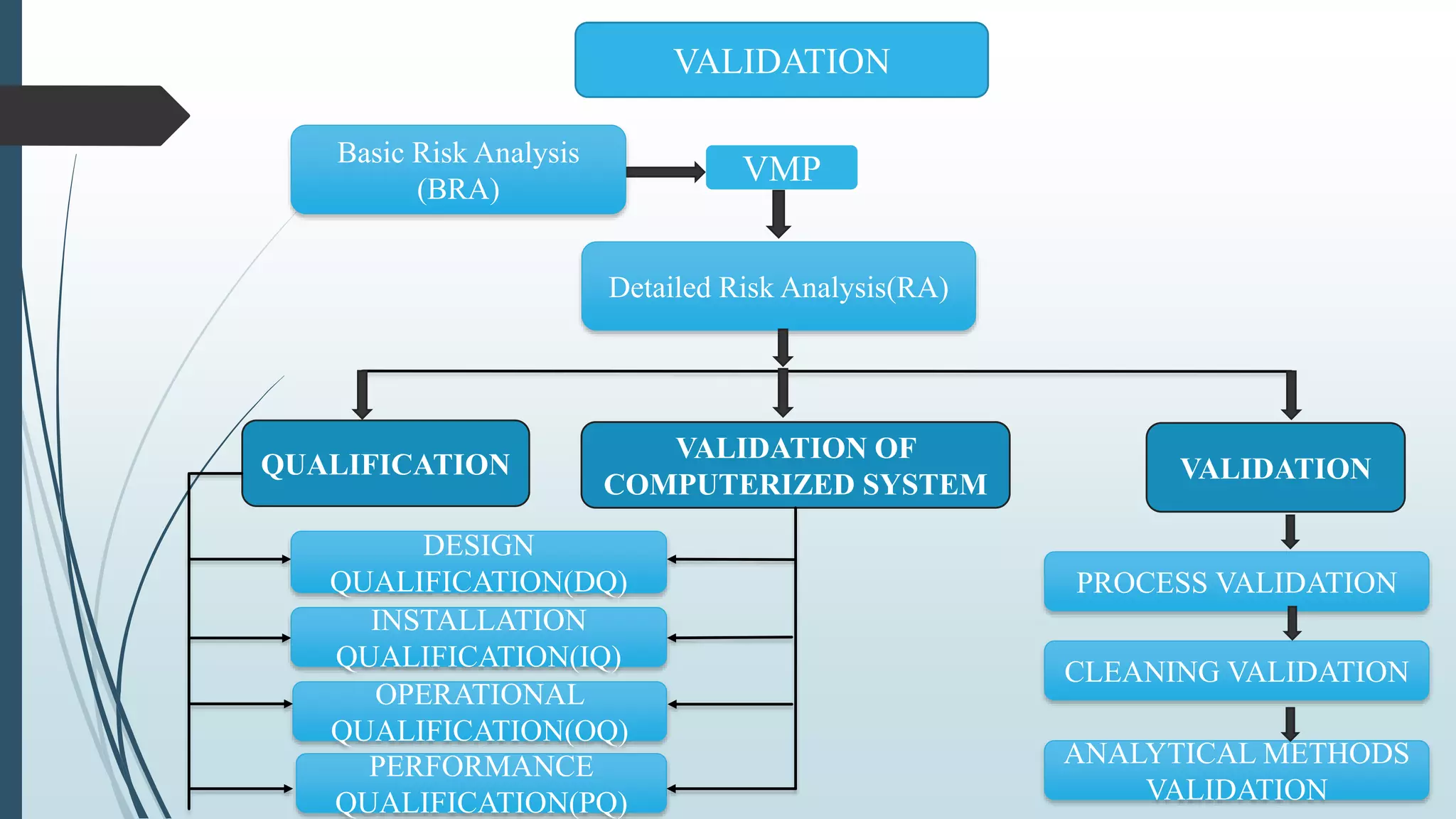
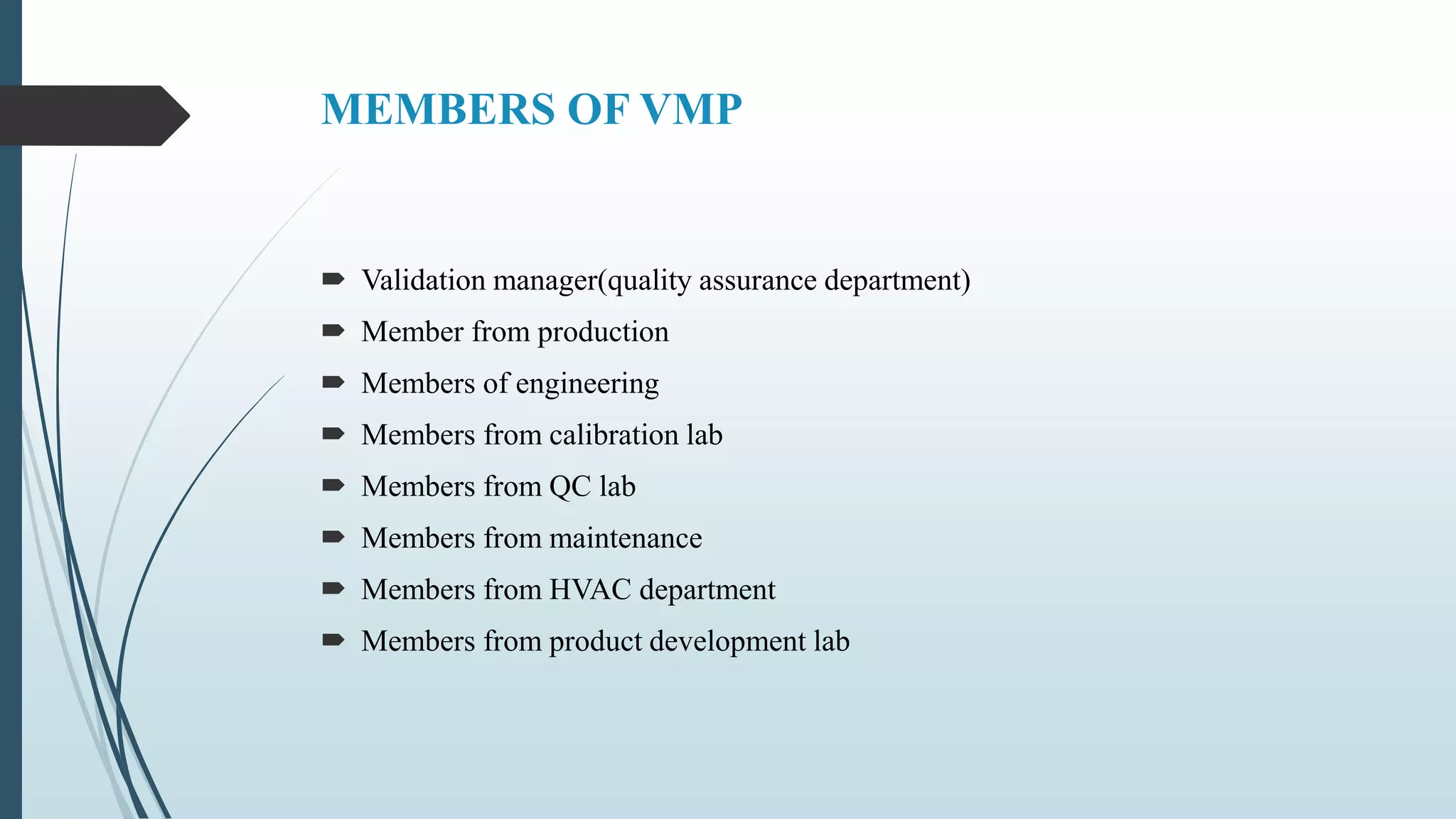
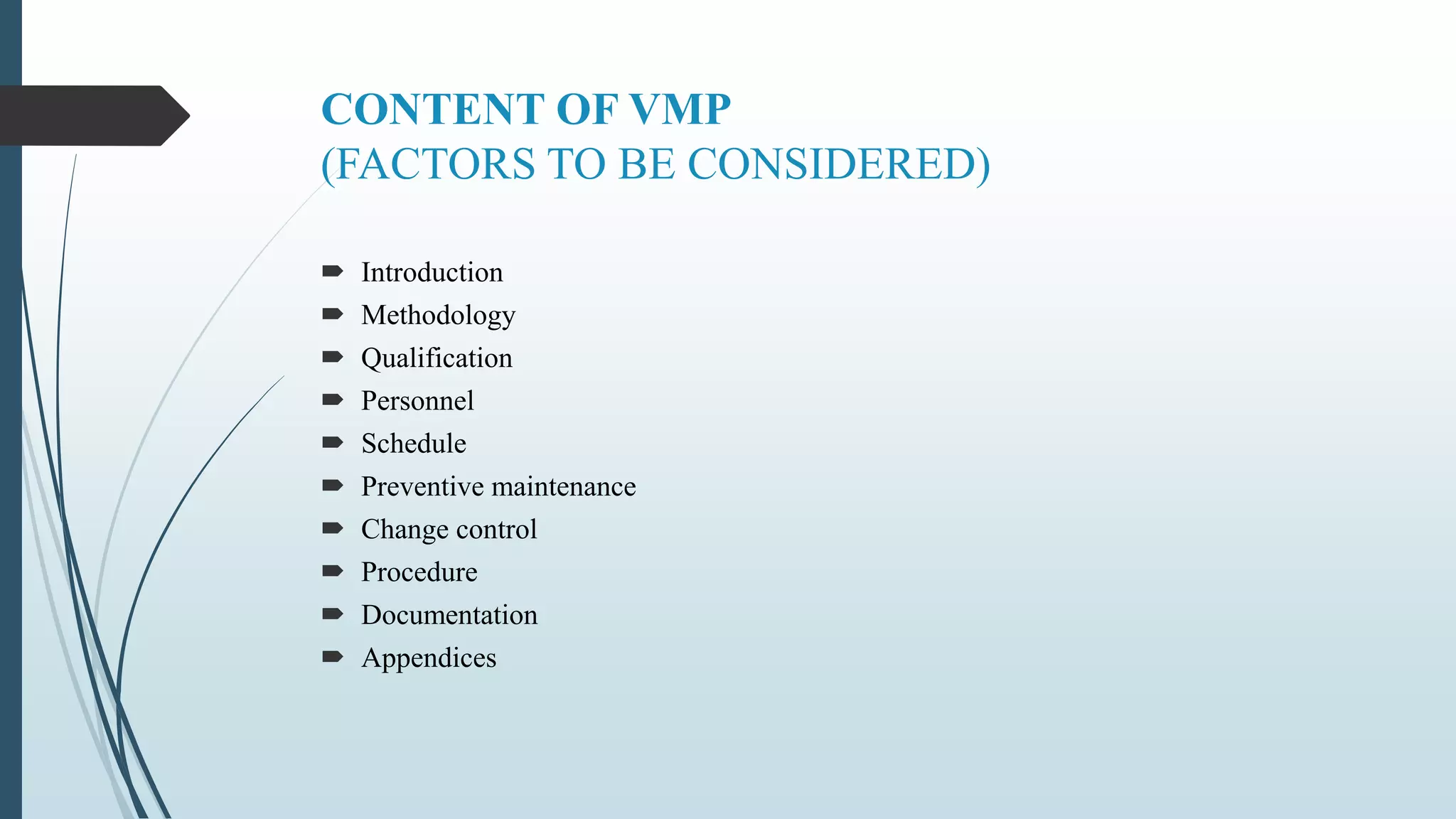
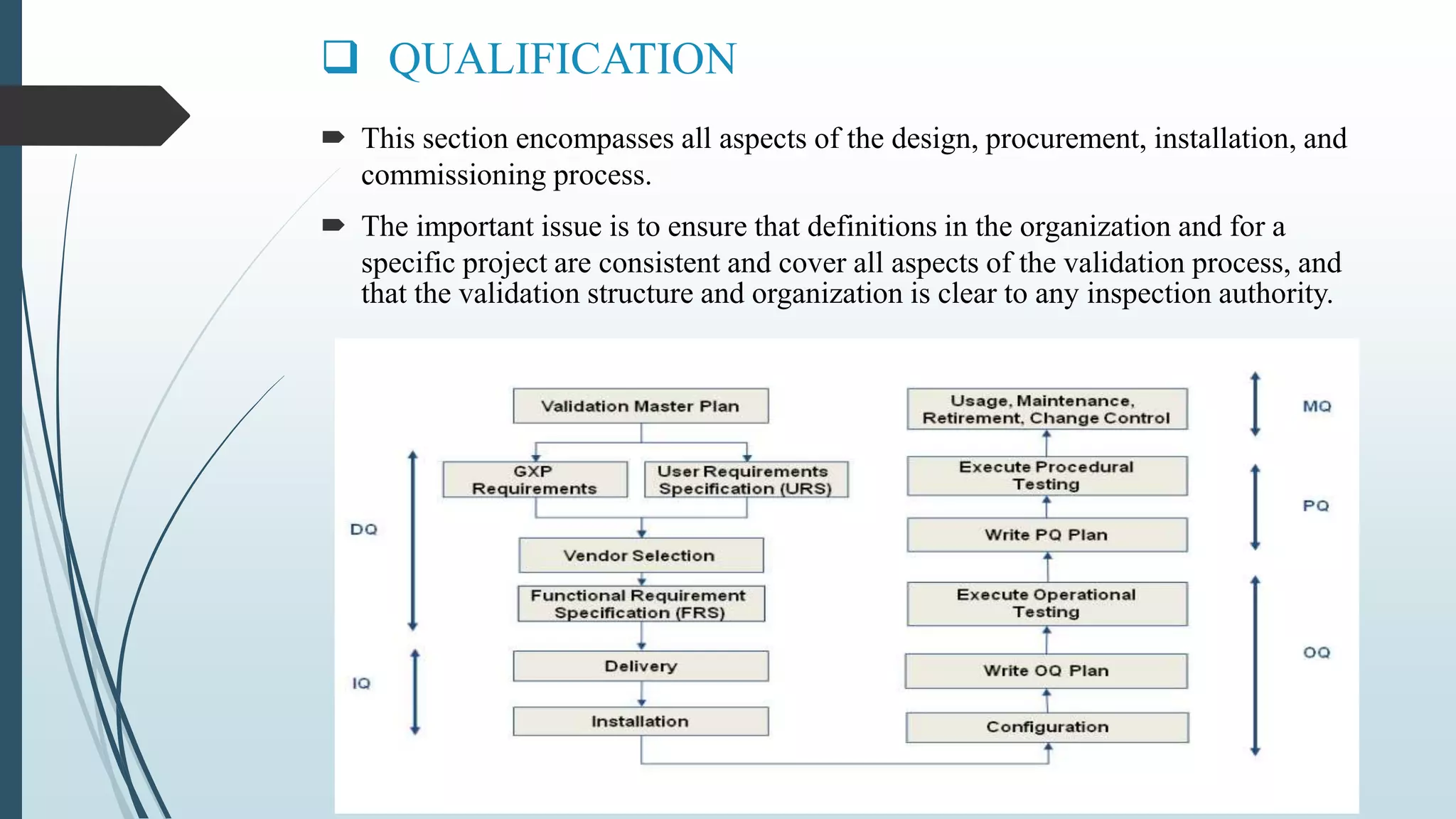
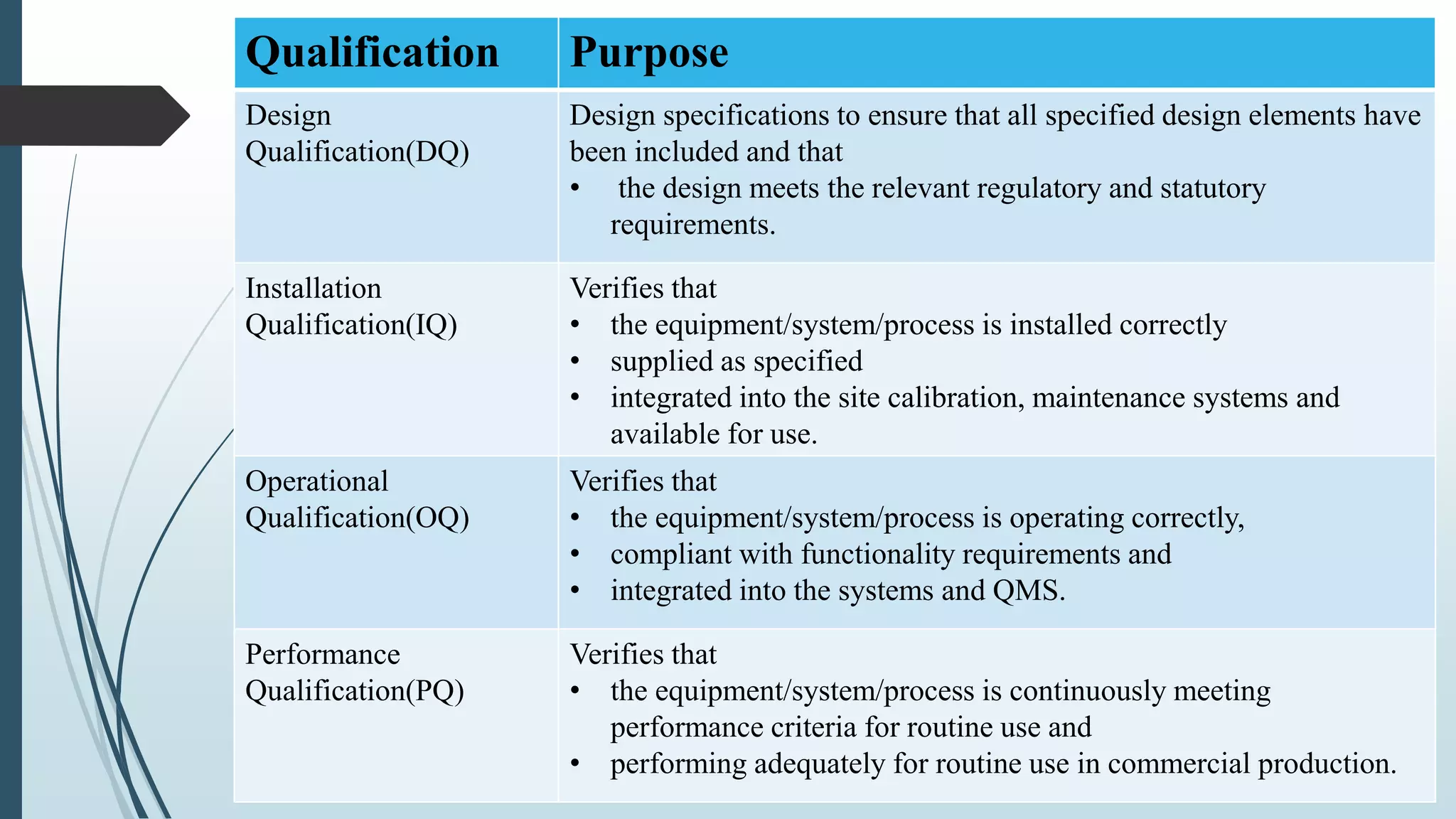
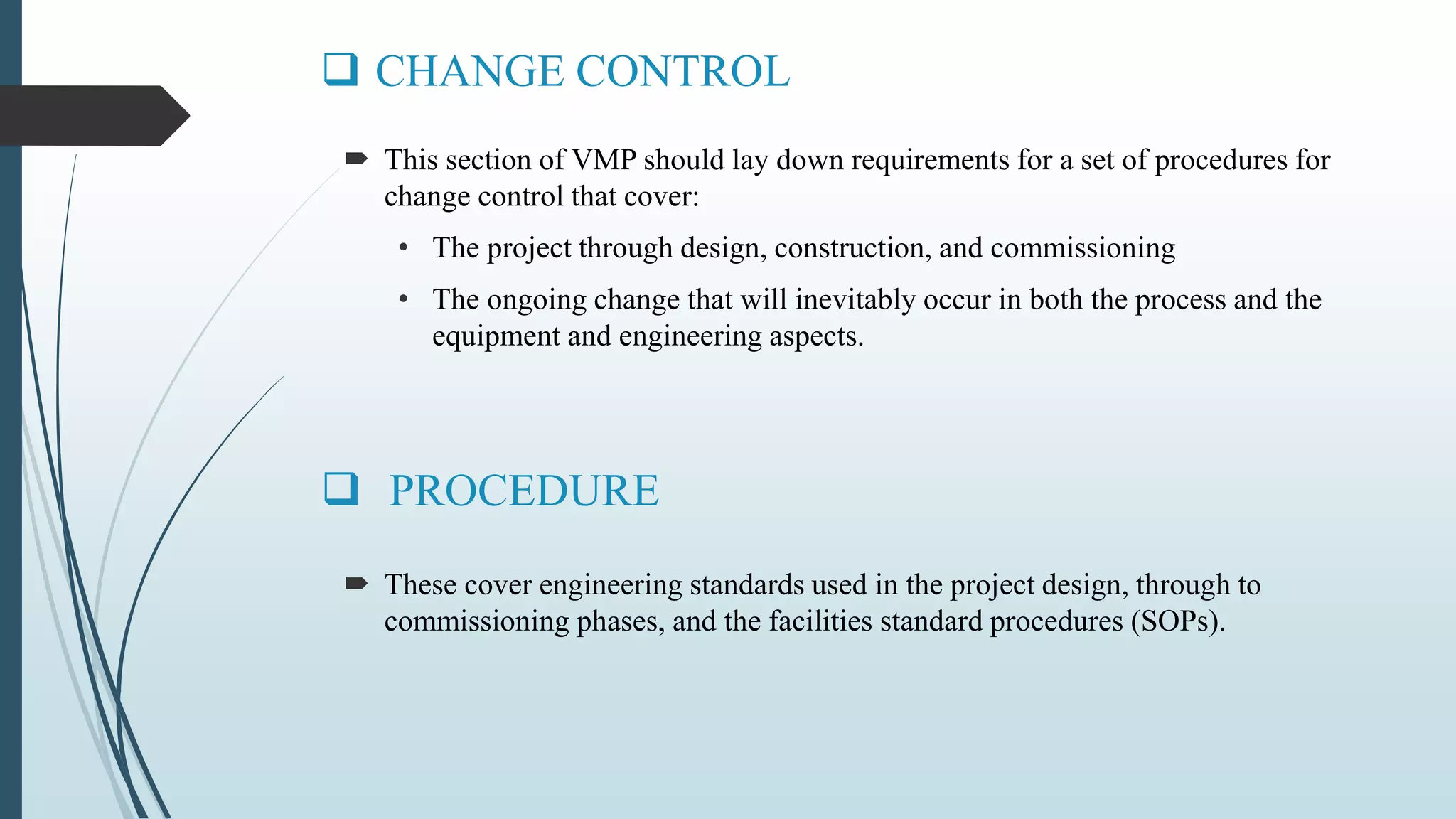
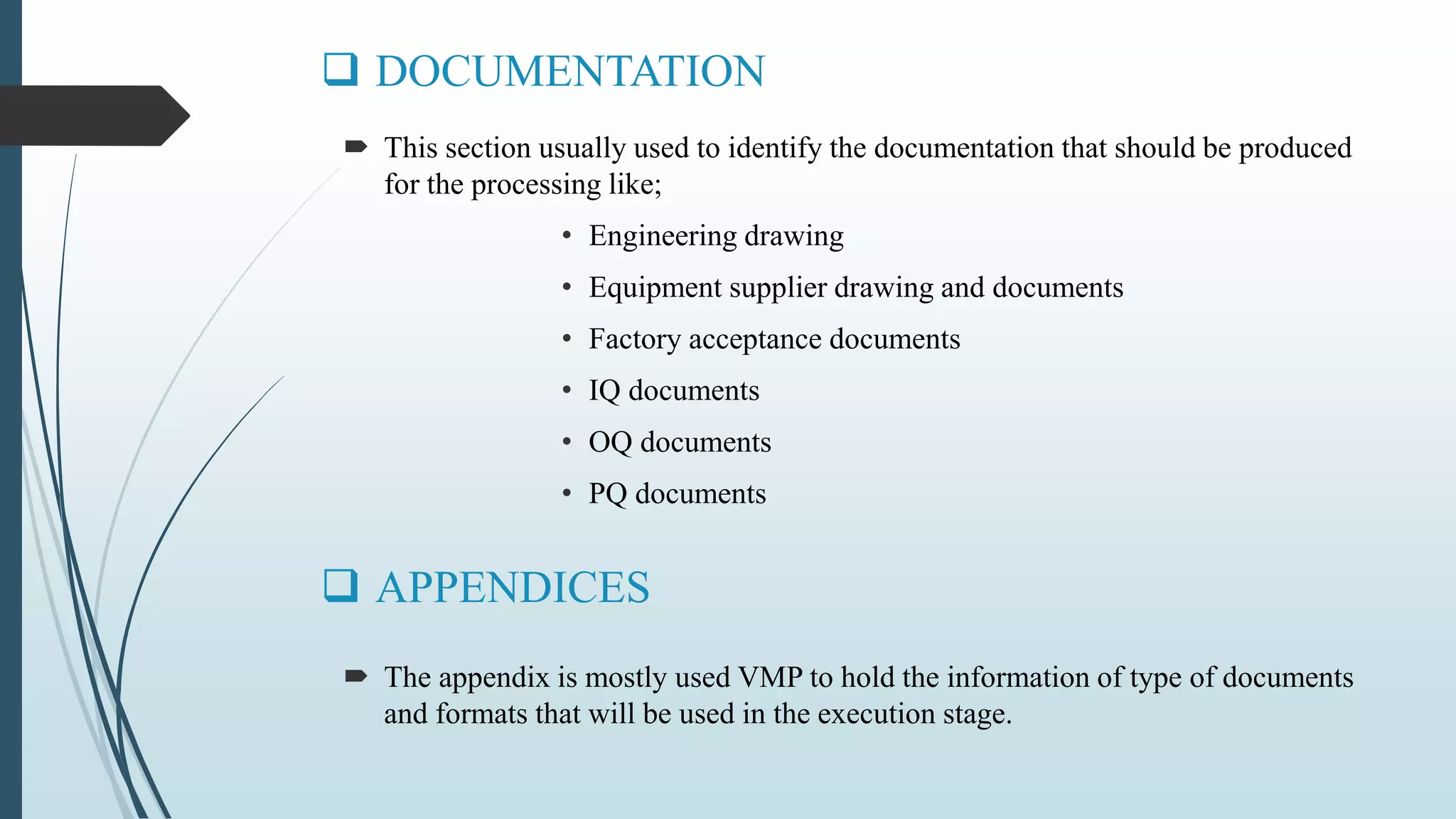
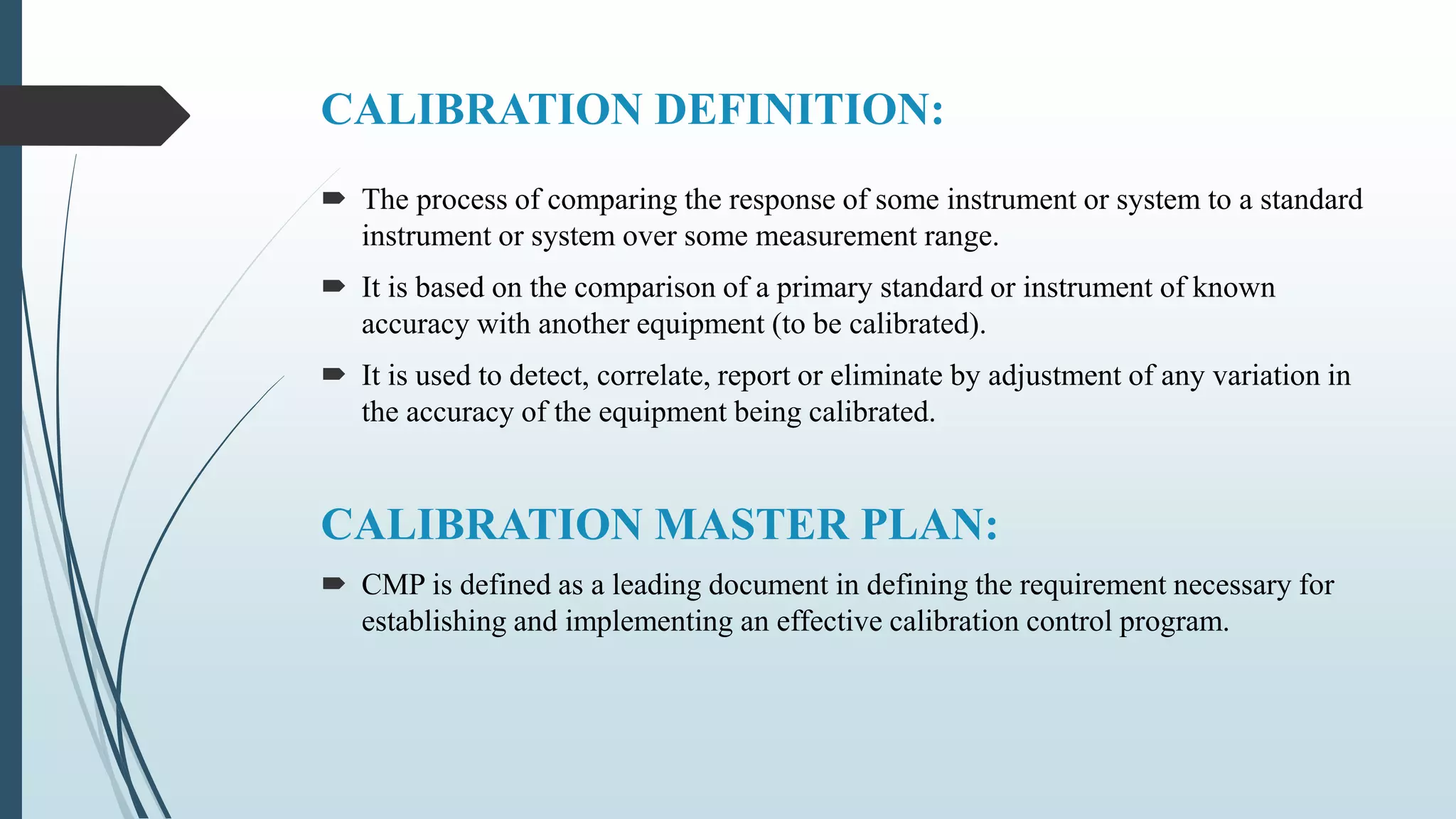
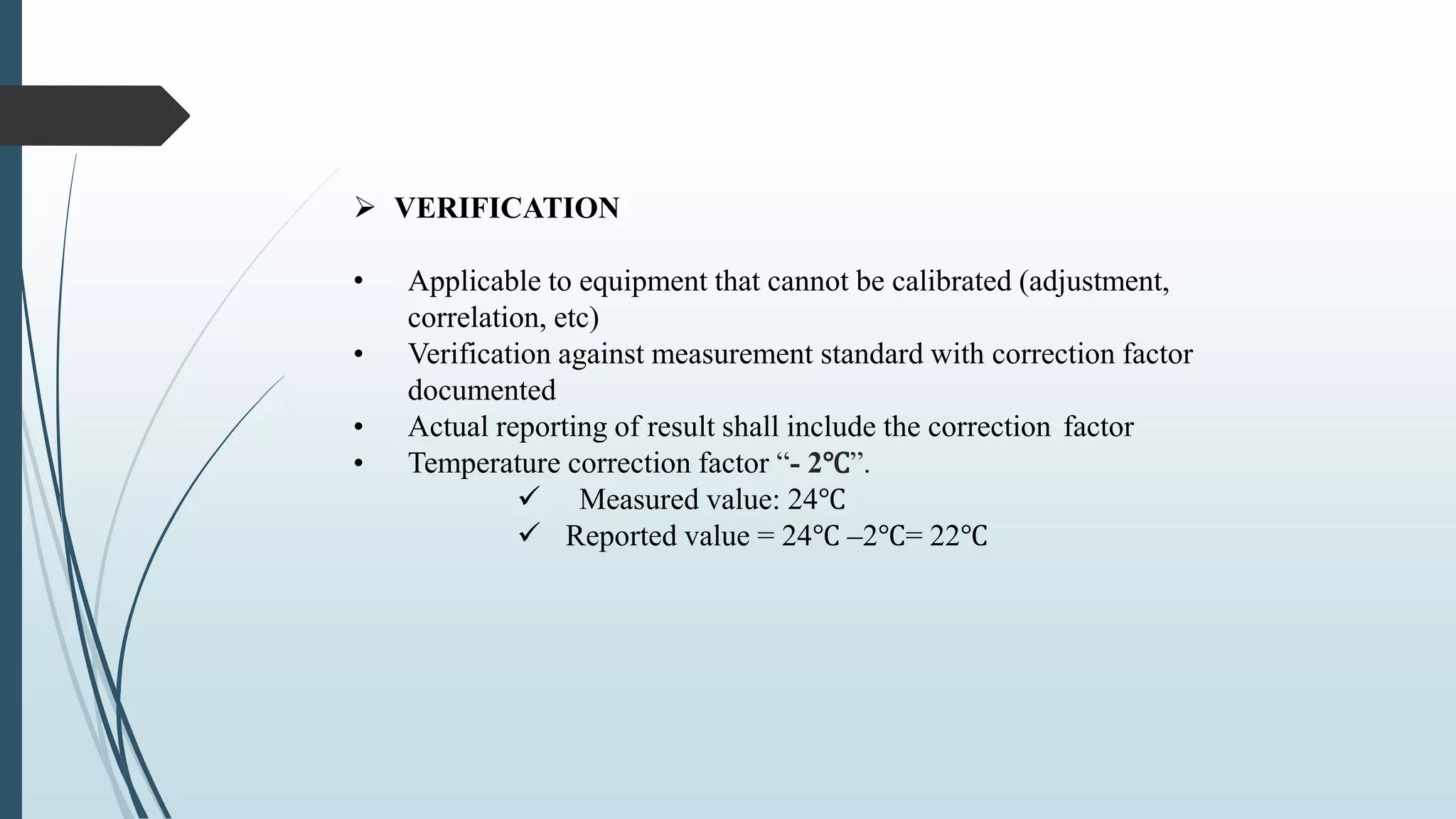
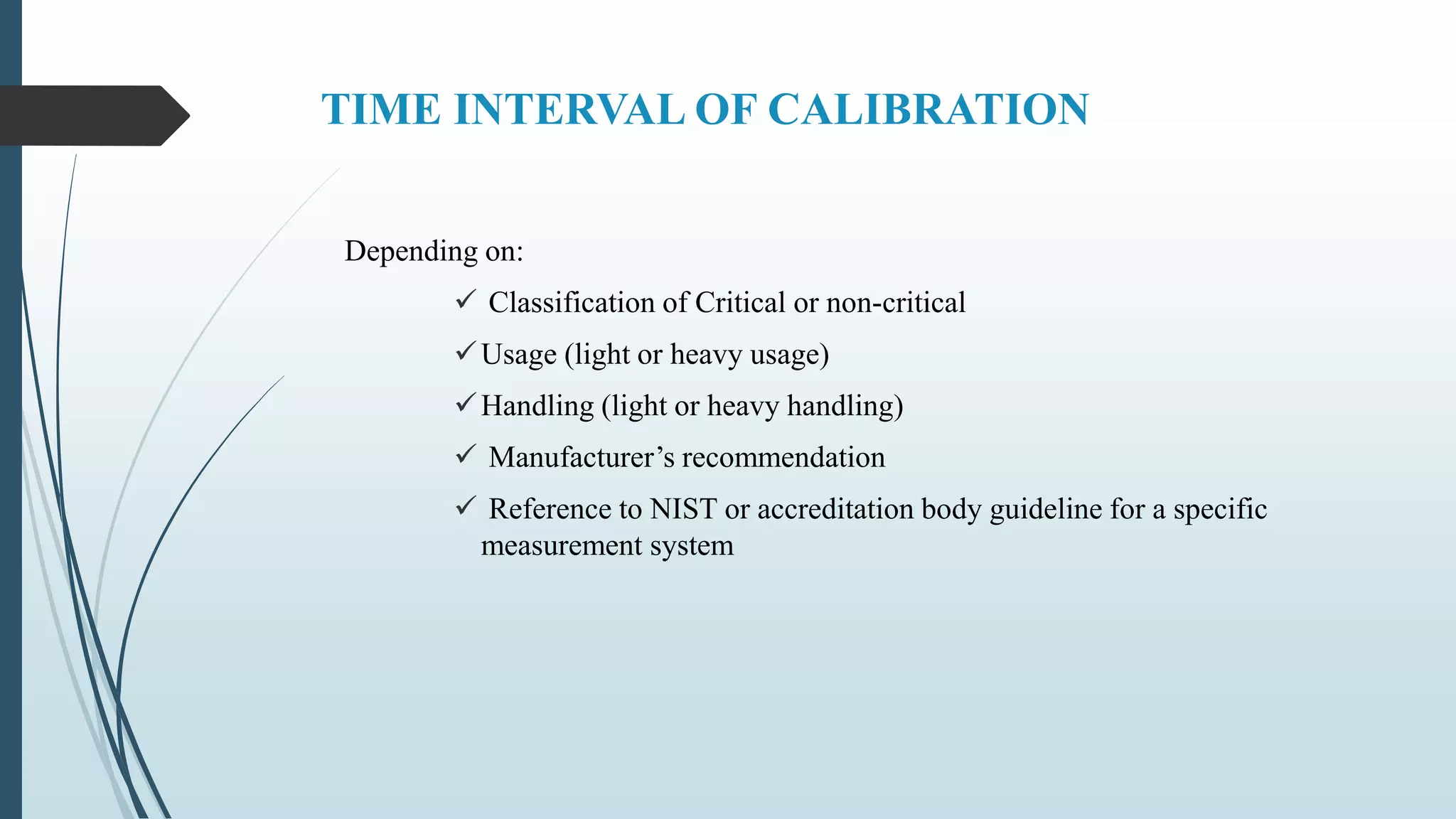
This document outlines validation and calibration master plans. It discusses the objectives of validation including reducing risks and costs. It describes the contents and members involved in a validation master plan, which provides the framework for validation activities. It also discusses the calibration process, including defining calibrated equipment, classification, and verification. The calibration master plan establishes requirements for an effective calibration control program.