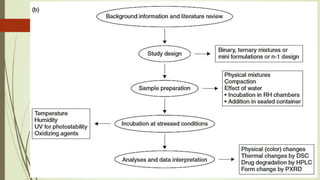
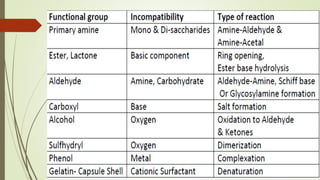
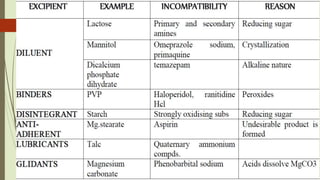
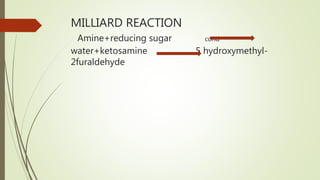
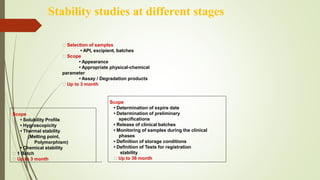
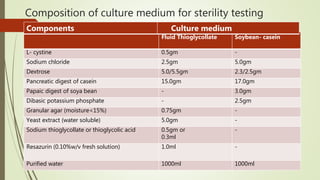
This document discusses modern pharmaceutics and preformulation concepts. It begins with an introduction to preformulation, which involves investigating a drug's physical and chemical properties alone and with excipients. This information guides dosage form development. The document then discusses drug-excipient interactions and compatibility testing methods. It also covers topics like solid dispersions, emulsions, suspensions, and parenteral product formulation and testing requirements.












![Theories of dispersion
Solid – Dispersion System
Definition
1. Solid dispersion is defined as dispersion of one or more active ingredients in an inert carrier or matrix at solid state prepared
by the melting, solvent or melting solvent method.
1. Molecular diffusion: obeys ficks first law and 2nd law of diffusion
2. Fick's first law relates the diffusive flux to the concentration under the assumption
of steady state.
3. J= -D(dS / dx)
4. j= flux, D = diffusivity , S= is concentration
5.
Fick's second law predicts how diffusion causes the concentration to change with
time. It is a partial differential equation which in one dimension reads:
where
•φ is the concentration in dimensions of [(amount of substance) length−3], example
mol/m3; φ = φ(x,t) is a function that depends on location x and time t
•t is time [s]
•D is the diffusion coefficient in dimensions of [length2 time−1], example m2/s
•x is the position [length], example m](https://image.slidesharecdn.com/preformulationconcept-171109153049/85/Preformulation-concept-18-320.jpg)
![Fick's second law predicts how diffusion causes the
concentration to change with time. It is a partial differential
equation which in one dimension reads:
dY/dT= D (d2Y/d2x)
where
•φ is the concentration in dimensions of [(amount of
substance) length−3], example mol/m3; φ = φ(x,t) is a function
that depends on location x and time t
•t is time [s]
•D is the diffusion coefficient in dimensions of [length2 time−1],
example m2/s
•x is the position [length], example m](https://image.slidesharecdn.com/preformulationconcept-171109153049/85/Preformulation-concept-19-320.jpg)



![ Instability[edit]
Emulsion stability refers to the ability of an emulsion to resist change in its
properties over time. There are four types of instability in
emulsions: flocculation, creaming, coalescence, and Ostwald ripening. An
everyday example of Ostwald ripening is the re-crystallization of water
ice cream which gives old ice cream a gritty, crunchy texture. Larger ice
crystals grow at the expense of smaller ones within the ice cream, creating
coarser texture
Flocculation occurs when there is an attractive force between the droplets,
they form flocs, like bunches of grapes. Coalescence occurs when droplets
bump into each other and combine to form a larger droplet, so the
droplet size increases over time. Emulsions can also undergo creaming,
where the droplets rise to the top of the emulsion under the influence
of buoyancy, or under the influence of the centripetal force induced when
a centrifuge is used.
An appropriate "surface active agent" (or "surfactant") can increase the
kinetic stability of an emulsion so that the size of the droplets does not
change significantly with time. It is then said to be stable.](https://image.slidesharecdn.com/preformulationconcept-171109153049/85/Preformulation-concept-23-320.jpg)





















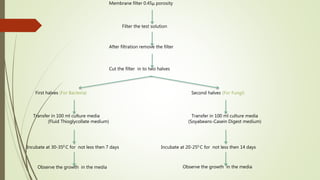






![Limulus amebocyte lysate [LAL] test
Limulus amebocyte lysate [LAL] test another method for the
determination of pyrogenic endotoxins
In this method the test solution is combined with a cell lysate from
the ameabocyte [blood cells] of horse shoe crab
Any endo toxin that might be present will be coagulated with
protien fraction of the ameabocytes and results in the formation
of a gel
This consider to be simple,rapid and of greater sensitivity that the
rabbit test](https://image.slidesharecdn.com/preformulationconcept-171109153049/85/Preformulation-concept-54-320.jpg)


