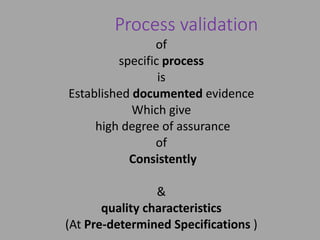
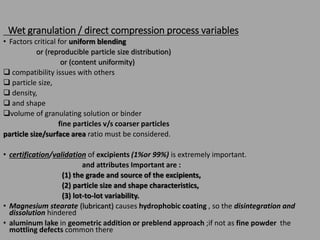
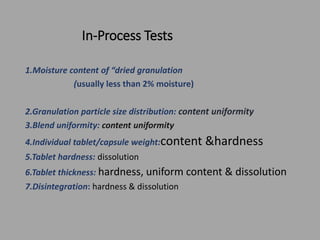
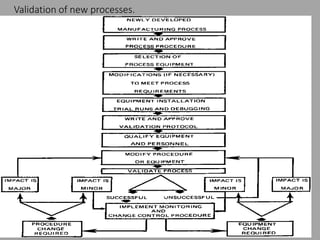
The document outlines the process validation of raw materials and manufacturing processes, emphasizing the importance of adherence to regulatory standards such as the FDA's current good manufacturing practices (cGMPs). It details steps for validating raw materials, methods for ensuring product quality through statistical controls, and the significance of evaluating process variables to achieve consistent product characteristics. Key elements include specification establishment, testing methodologies, and the necessity of both in-process and finished product testing to maintain quality assurance.