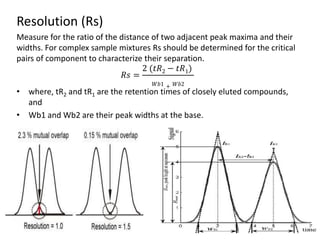
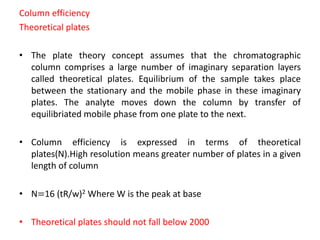
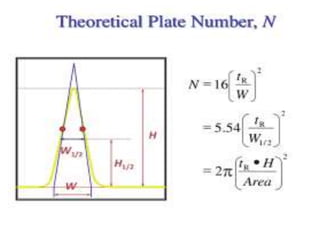
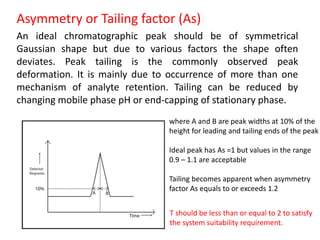
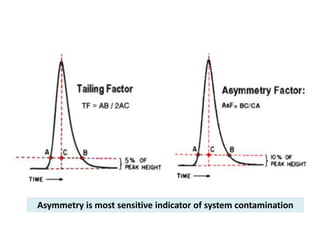
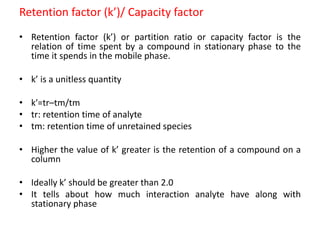
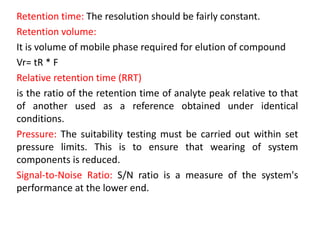
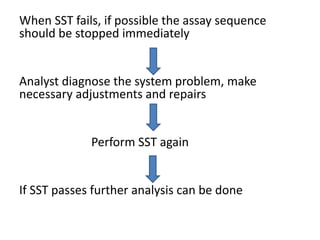
System suitability tests (SST) ensure that chromatography systems are operating as expected prior to sample analysis. Key parameters evaluated include column efficiency, resolution, asymmetry, retention time, capacity factor, and precision. SST solutions contain analytes of interest and closely eluting components at known levels. Acceptance criteria are established during validation. If an SST fails, the analysis is stopped and the system diagnosed before re-running the SST. SSTs help reduce unnecessary retesting of samples by identifying potential issues early.