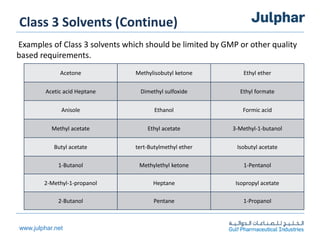
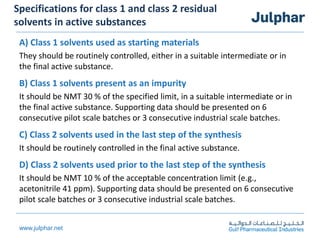
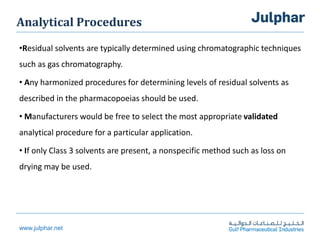
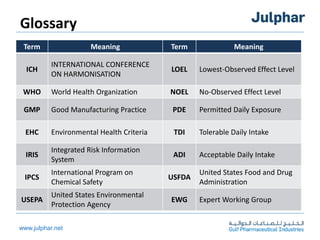
The document provides an overview of the ICH Q3C guideline for residual solvents. It classifies residual solvents into 3 classes based on risk: Class 1 solvents to be avoided, Class 2 solvents to be limited, and Class 3 solvents with low toxic potential. Limits are defined for each class, with Class 1 solvent limits being the strictest. Options for describing limits of Class 2 solvents include assuming a daily dose of 10g or adding amounts in components. Analytical procedures and reporting levels are also outlined. The goal is to recommend safe levels of residual solvents to protect patient safety.