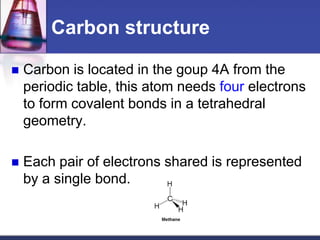
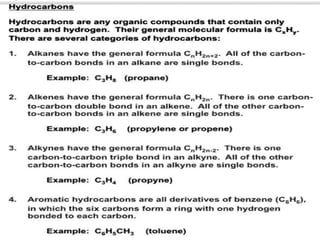
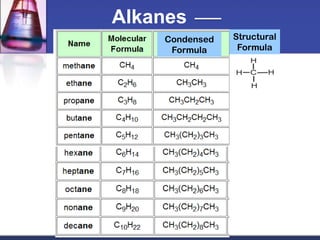
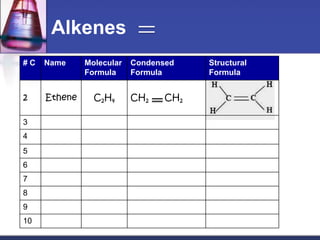
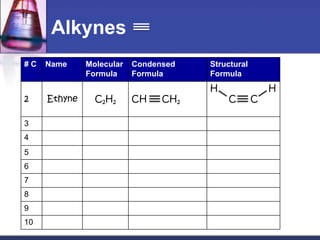
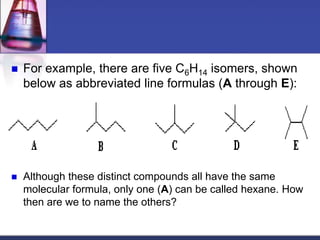
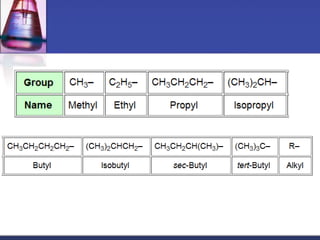
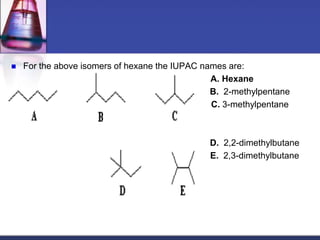
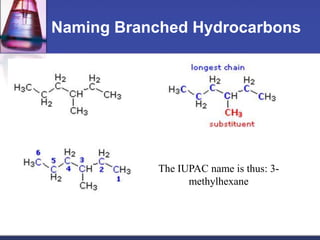
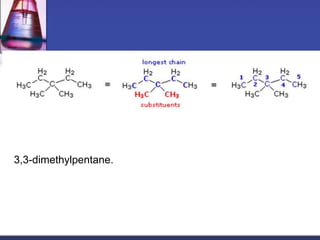
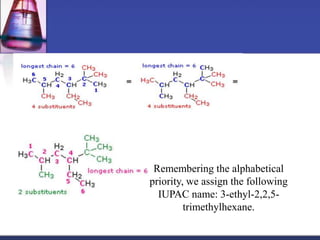
This document discusses hydrocarbons and their derivatives. It begins by defining organic compounds and their main classes - hydrocarbons and hydrocarbon derivatives. Hydrocarbons contain only carbon and hydrogen and can be straight-chain, branched-chain, or cyclic. The document then discusses hydrocarbon nomenclature, including naming conventions for alkanes, alkenes, alkynes, and branched hydrocarbons. It concludes by mentioning some common hydrocarbon derivatives like halides, alcohols, and ethers and instructing the reader to investigate their general formulas, functional groups, and properties.