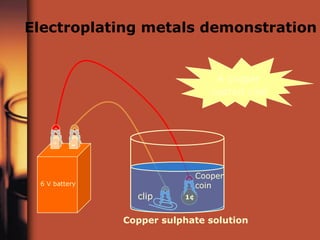
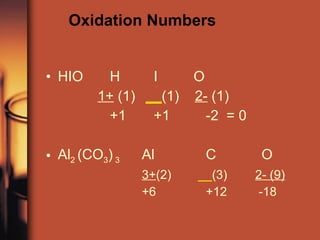
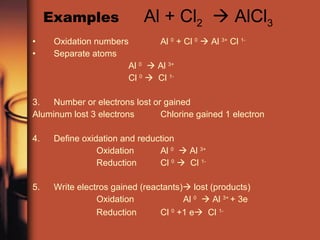
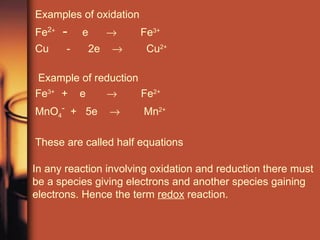
The document discusses oxidation and reduction reactions. It provides examples of oxidation as the loss of electrons and reduction as the gain of electrons. Corrosion is described as an oxidation reaction where metals react with substances like oxygen, water and acids. Electroplating is introduced as a process where a thin coating of a metal like copper or nickel is deposited onto a surface through an oxidation-reduction reaction.