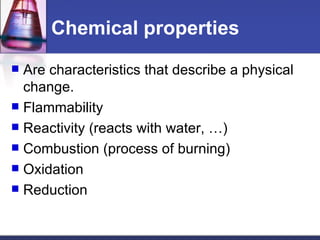
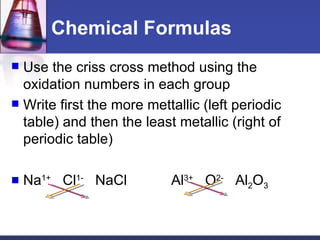
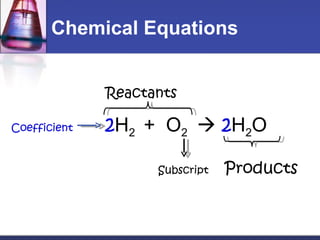
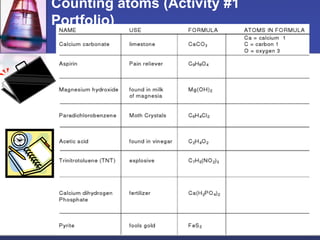
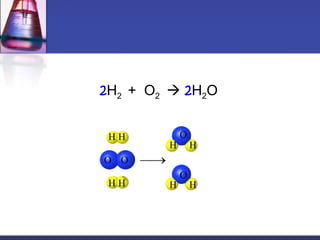
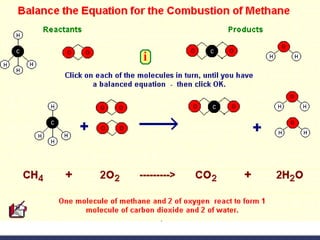
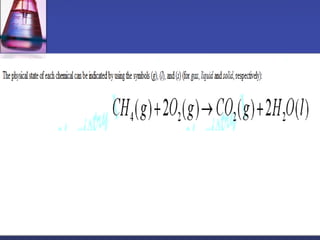
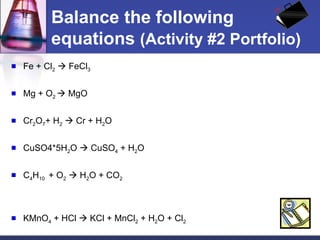
This document provides an overview of chemistry concepts including physical and chemical changes, physical and chemical properties, chemical formulas, chemical equations, and balancing chemical equations. It defines physical changes as changes in state or phase that do not produce a new substance, while chemical changes occur at the molecular level and produce new substances. It also discusses using coefficients to balance chemical equations so that the same number and type of atoms are on both sides according to the law of conservation of mass.