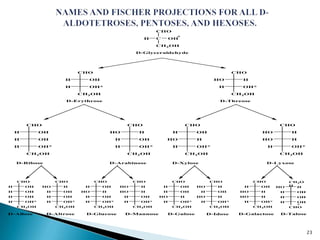
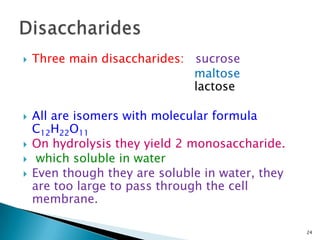
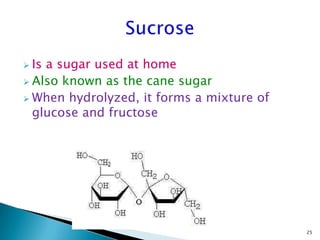
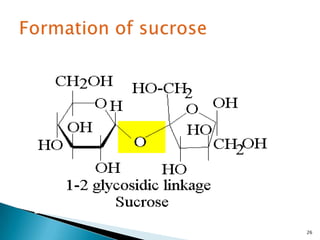
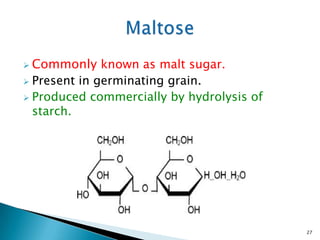
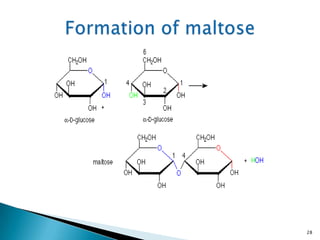
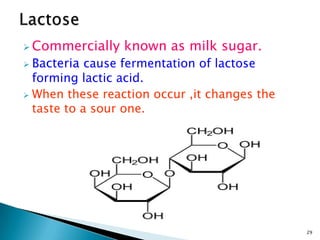
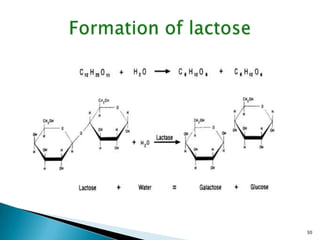
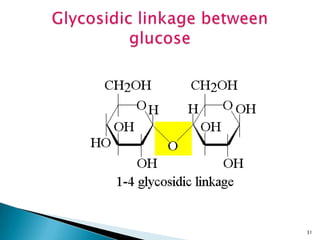
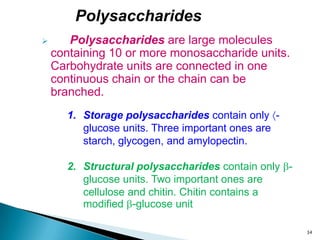
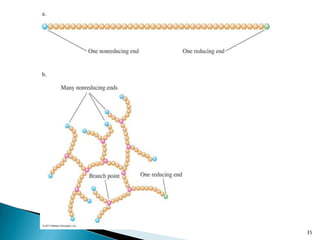
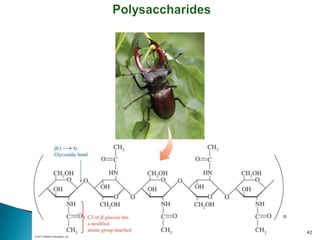
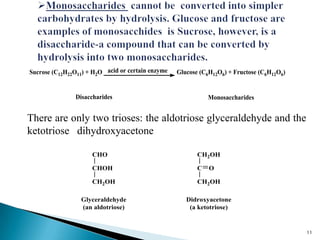
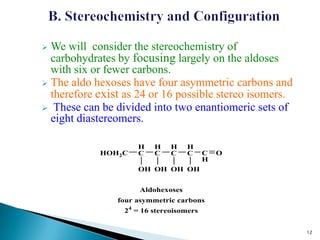
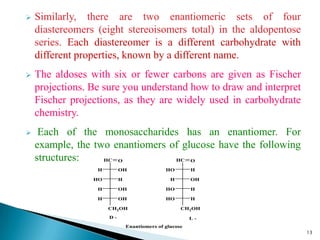
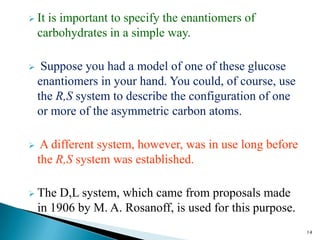
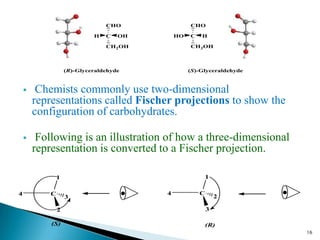
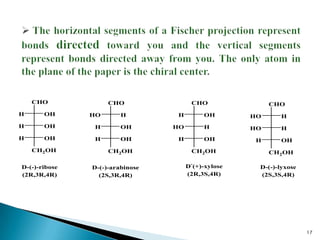
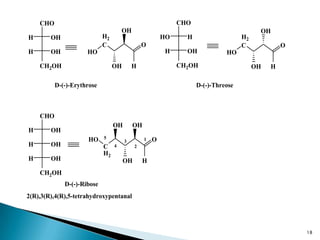
This document provides an overview of carbohydrates. It begins by defining carbohydrates as the most abundant organic compounds in plants, acting as energy stores and structural components. It then discusses monosaccharides, disaccharides, and polysaccharides. Specific carbohydrates discussed include glucose, fructose, sucrose, maltose, lactose, starch, glycogen, cellulose, and chitin. It explains their structures, functions, and important properties. The document is a comprehensive introduction to carbohydrate chemistry.









![ Fischer could have been wrong, but by a stroke of good
fortune he was correct, as proven in 1952 by a special
application of X-ray crystallography.
D- and L-glyceraldehyde serve as reference points for the
assignment of relative configuration to all other aldoses and
ketoses.
CHO
CH OH
CH2OH
D-Glyceraldehyde
[]D = +13.5
CHO
C HHO
CH2OH
25
L-Glyceraldehyde
[]D = -13.525
21](https://image.slidesharecdn.com/carbohydrateppt-140417063725-phpapp01/85/Carbohydrate-ppt-21-320.jpg)