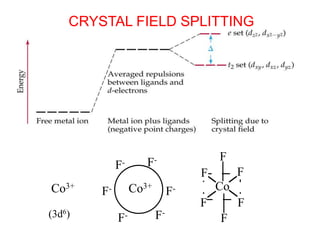
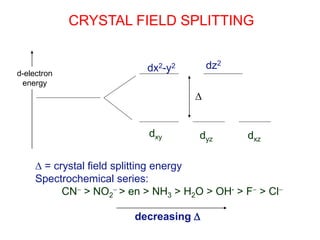
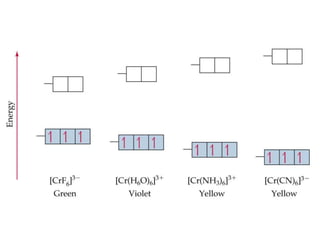
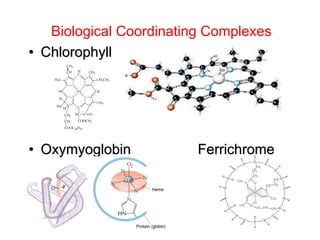
This document provides information about transition metals and their coordination compounds. It discusses the key characteristics of transition metals, including having multiple oxidation states and forming colored compounds. Transition metals form coordination complexes by acting as Lewis acids and coordinating with ligands that donate lone pair electrons. The document explains crystal field theory and how ligands of different strengths cause splitting of the d-orbital energies, resulting in different colors for coordination complexes.



![ELECTRON CONFIGURATIONS
3d elements: Sc Zn
Ar 3s23p6 Sc [Ar]3d14s2
K [Ar]4s1 Ti [Ar]3d24s2
Ca [Ar]4s2 . .
. .
. .
Zn [Ar]3d104s2
Note: 4s is filled before 3d, but when oxidized, 4s electrons are lost
before 3d.
Ti [Ar]3d24s2
Ti2+ [Ar]3d24s0
Ti3+ [Ar]3d14s0
Ti4+ [Ar]3d04s0
Ti5+ does not exist!](https://image.slidesharecdn.com/transitionmetals-161214195324/85/Transition-metals-4-320.jpg)
![TRANSITION METALS: ScMn
Oxidation States:
Highest oxidation states of Sc, Ti, V, Cr, Mn = number of
valence (4s + 3d) electrons.
Sc [Ar]3d14s2 Sc3+ [Ar] maximum
Mn [Ar]3d54s2 Mn7+[Ar] maximum
Trend from Sc Mn:
The max. oxidation state becomes increasingly unstable.
Sc3+, Ti4+ are stable (maximum oxidation states).
Sc2O3 Stable oxide.
Mn7+ Exists but is easily reduced.
MnO4
- Strong oxidizing agent.
Transition Metals](https://image.slidesharecdn.com/transitionmetals-161214195324/85/Transition-metals-5-320.jpg)


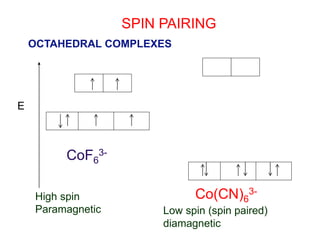
![Complex -
•Metal- Ligand compounds
• [MLn] i.e., [Ag(NH3)2]+ or [Co(NH3)6] Cl3
•[ ] denotes atoms bonded to each other
through covalent bonds. These atoms are
contained in the coordination sphere.
Coordinated sphere is the directly bonded to each other.
Counter ions are outside bracket, and are not part of the coordinate
sphere.
A coordinated compound behaves like an electrolyte in water: the
complex ion and counter separates from each other. But the complex
ion behaves like a polyatomic ion: the ligands and central metal ion
remain attached.](https://image.slidesharecdn.com/transitionmetals-161214195324/85/Transition-metals-9-320.jpg)
![Coordinated Complexes and Coordination
Number
•Coord Shape Example
•Number
• 2 Linear [CuCl2]-, [Ag(NH3)2]+, [AuCl2]-
• 4 Square Planar [Ni(CN)4] 2-, [PdCl4]2-
• [Pt(NH3)4] 2+, [Cu(NH3)4] 2+
• 4 Tetrahedral [Cu(CN)4] 3-, [Zn(NH3)4]2+
• [CdCl4] 2-, [MnCl4] 2-
• 6 Octahedral [Cu(H2O)6] 3+, [V(CN)6] 4-,
• [Cu(NH3)4Cl2] +, [Co(en)3] 3+
F
F
Br
F
F
F
F
F
S
F
F
F](https://image.slidesharecdn.com/transitionmetals-161214195324/85/Transition-metals-10-320.jpg)
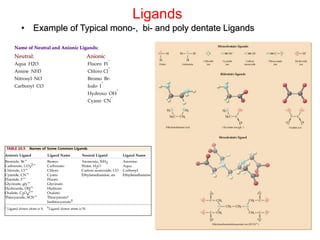
![Chelates
• Chelating Ligands have two or more donor atoms that
simultaneously coordinate to a single metal ion.
• Polydentate - (Many toothed - ligand)
• Chelating agent (Claw)
• Sequestering agent - sequester - to set apart or
separate
• en ethylenediamine (shown) - two toothed ligand:
– i.e., [Co(en)3]3+ [Pt(en)2]2+
• EDTA ethylenediaminetetraacetate
– (picture) hexadentate
– EDTA is the antidote for
– heavy metal poisoning](https://image.slidesharecdn.com/transitionmetals-161214195324/85/Transition-metals-12-320.jpg)
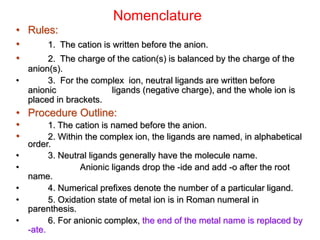
![Nomenclature
• Cation - Anion: Salts: name cation before anions i.e.,
[Co(HN3)5Cl]Br2, we name [Co(HN3)5Cl] complex ion before bromides
counter ions .
• Complex: Within complex ion, the ligands are named in alphabetical
order before the metal i.e., pentaaminechlorocobalt(II), note that tetra is
an indication of the number of NH3 group, and not considered in the
alphabetizing of the ligand.
• Ligand: Anionic ligands end in -o and neutral ligands are name based
on their molecular name (excepts are aqua H2O, amine: NH3)
–Greek prefixes are used to indicate number of ligands, di-, tri-, tetra-,
penta-, hexa-. Exception occurs when ligand already has Greek prefix
in its name, The prefixes bis-, tris-, tetrakis-, pentakis, & hexakis. are
used instead.
– i.e., Ir(bpy)3 trisbipyridineiridium (III) bipyridine already has bi in its
name.
• If the complex is an anion, then its name ends with suffix -ate.
• Further more, oxidation state of the metal is given in roman numerals in
parenthesis at the end of the name.](https://image.slidesharecdn.com/transitionmetals-161214195324/85/Transition-metals-15-320.jpg)
![Example: Naming from Formula
• Name from formula
• a) K3[Au(CN)4]
• Potassium Tetracyanoaurate(I)
• d) K[Co(C2O4)2(NH3)2]
• Potassium diaminedioxaloCobaltate(I)
• f) [Cr(en)2F2]NO3
• Bis(ethylenediamine)difluorochromium(III) nitrate
• Naming anionic metals
• Iron: Ferrate Copper: Cuprate
• Lead: Plumbate Silver:
Argentate
• Gold: Aurate Tin: Stannate](https://image.slidesharecdn.com/transitionmetals-161214195324/85/Transition-metals-17-320.jpg)
![Example: Formula from Name
• Name from formula
• a) Hexaamminechromium(III) nitrate
• [Cr(NH3)6] (NO3)3
• d) dichlorobis(ethylenediamine)platinum(IV) bromide
• [PtCl2(en)2]Br2
• f) bis(ethylenediamine)zinc(II) tetraiodomercurate(II)
• [Zn(en)2][HgI4]
•
• More anionic metals
• Osmium: Osmate Cobalt: Cobaltate
• Amtimony: Antimonate Rhenium:
Rhenate
• Platinum: Platinate Rhodium: Rhodate](https://image.slidesharecdn.com/transitionmetals-161214195324/85/Transition-metals-18-320.jpg)