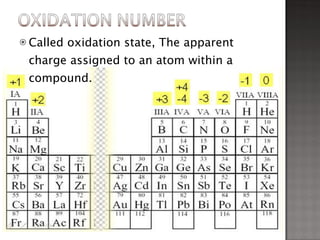
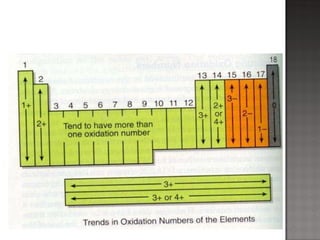
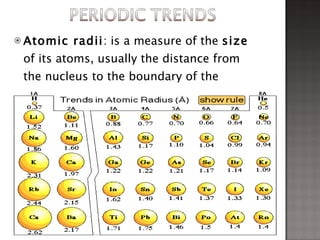
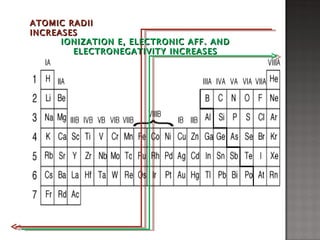
1. Johann Döbereiner noticed that the atomic weight of strontium fell between calcium and barium, elements with similar properties, proposing the Law of Triads.
2. John Newlands classified elements into groups and noted pairs differed by multiples of eight in atomic weight, proposing the Law of Octaves.
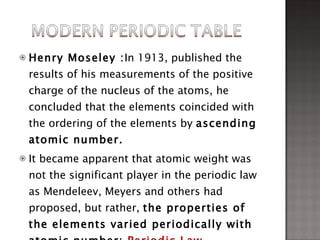
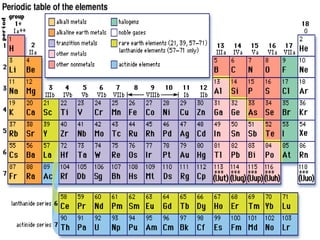
3. Dmitri Mendeleev developed the first recognizable periodic table, ordering elements by atomic mass and predicting undiscovered elements, though Henry Moseley later showed atomic number was fundamental.