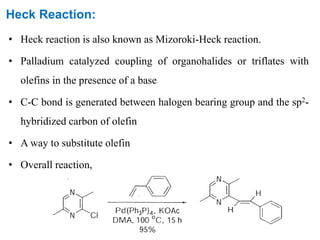
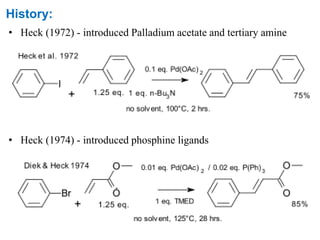
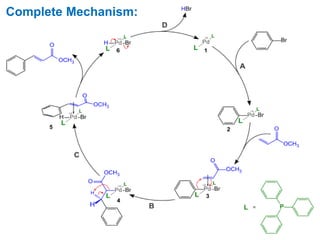
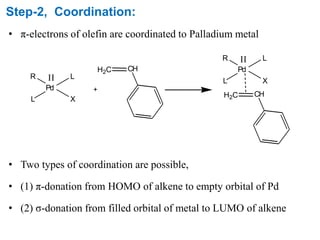
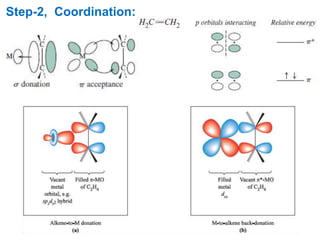
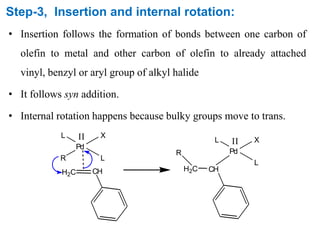
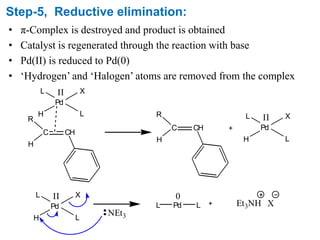
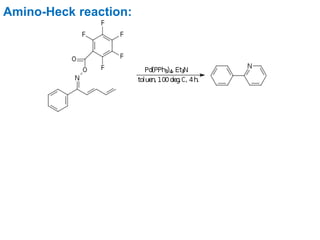
The Heck reaction is a palladium-catalyzed coupling reaction between an aryl or vinyl halide and an alkene, generating a new carbon-carbon bond. The reaction proceeds through oxidative addition, coordination, insertion, beta-hydride elimination and reductive elimination steps with a palladium catalyst and base. The Heck reaction is widely used in pharmaceutical synthesis such as for the NSAID Naproxen and in materials synthesis like for sunscreen agents.