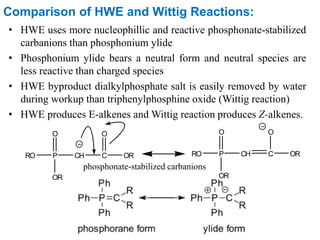
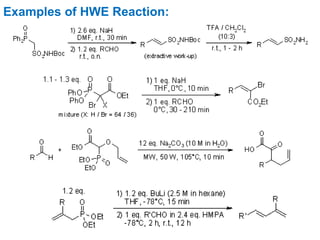
The Horner-Wadsworth-Emmons (HWE) reaction involves reacting a phosphonate with an aldehyde or ketone in the presence of a strong base to form an alkene. The reaction proceeds through a three step mechanism: 1) deprotonation of the methylene group of the phosphonate by the base, 2) reaction of the carbanion intermediate with the carbonyl group to form a tetrahedral intermediate, and 3) elimination of the phosphate group to form the alkene product. The HWE reaction is useful for synthesizing alkenes from carbonyl compounds and has advantages over the Wittig reaction such as producing E-alkene products and having an easier








![Bases and Solvent:
• Lithium hydride (LiH) or Sodium hydride (NaH)
• Due to sensitivity of substrates towards these hydrides, the bases
used are lithium chloride with 1,8-diazabicyclo[5.4.0]undec-7-ene
(DBU), lithium/magnesium halide with triethylamine (NEt3)
• Potassium carbonate (K2CO3) or Cesium carbonate (Cs2CO3) are
used as bases in solvent free synthesis
• One of the liquid reactants is in excess acting as solvent.
• Different solvents have also been reported including
dimethoxyethane (DME), tetrahydrofuran (THF) etc.
DBU](https://image.slidesharecdn.com/horner-wadsworth-emmonsreaction-200717164918/85/Horner-Wadsworth-Emmons-reaction-9-320.jpg)