Embed presentation
Downloaded 118 times


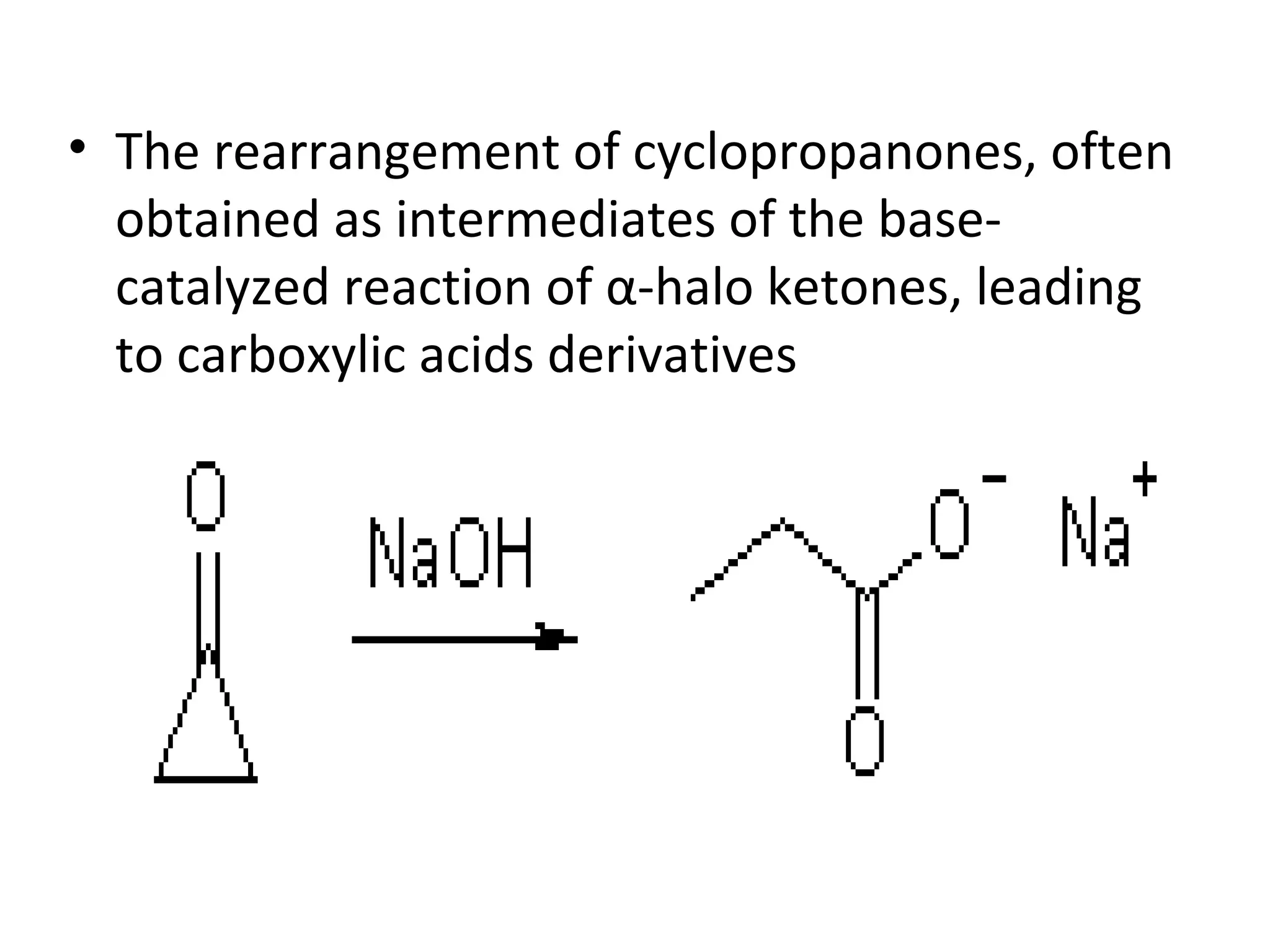
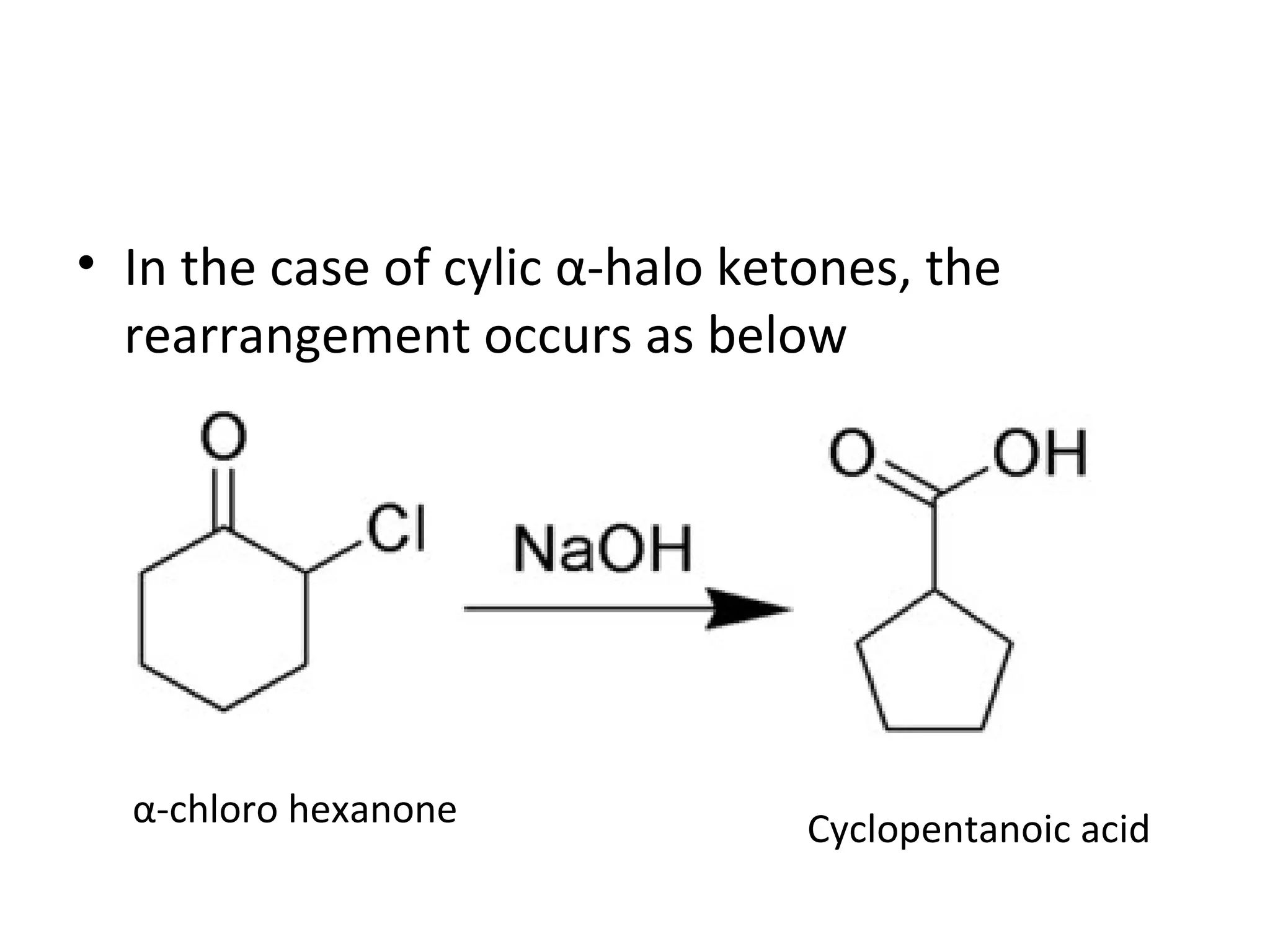
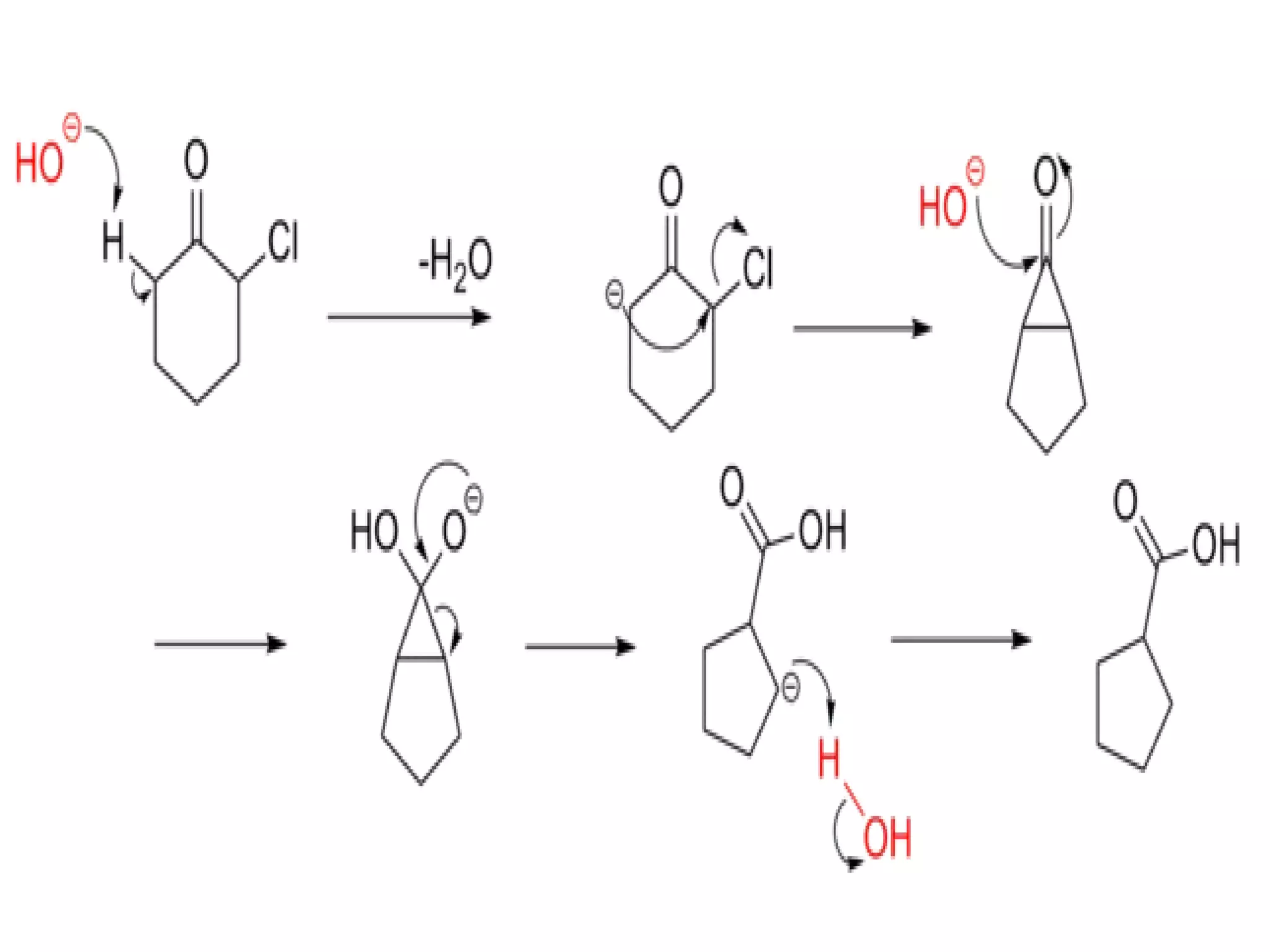

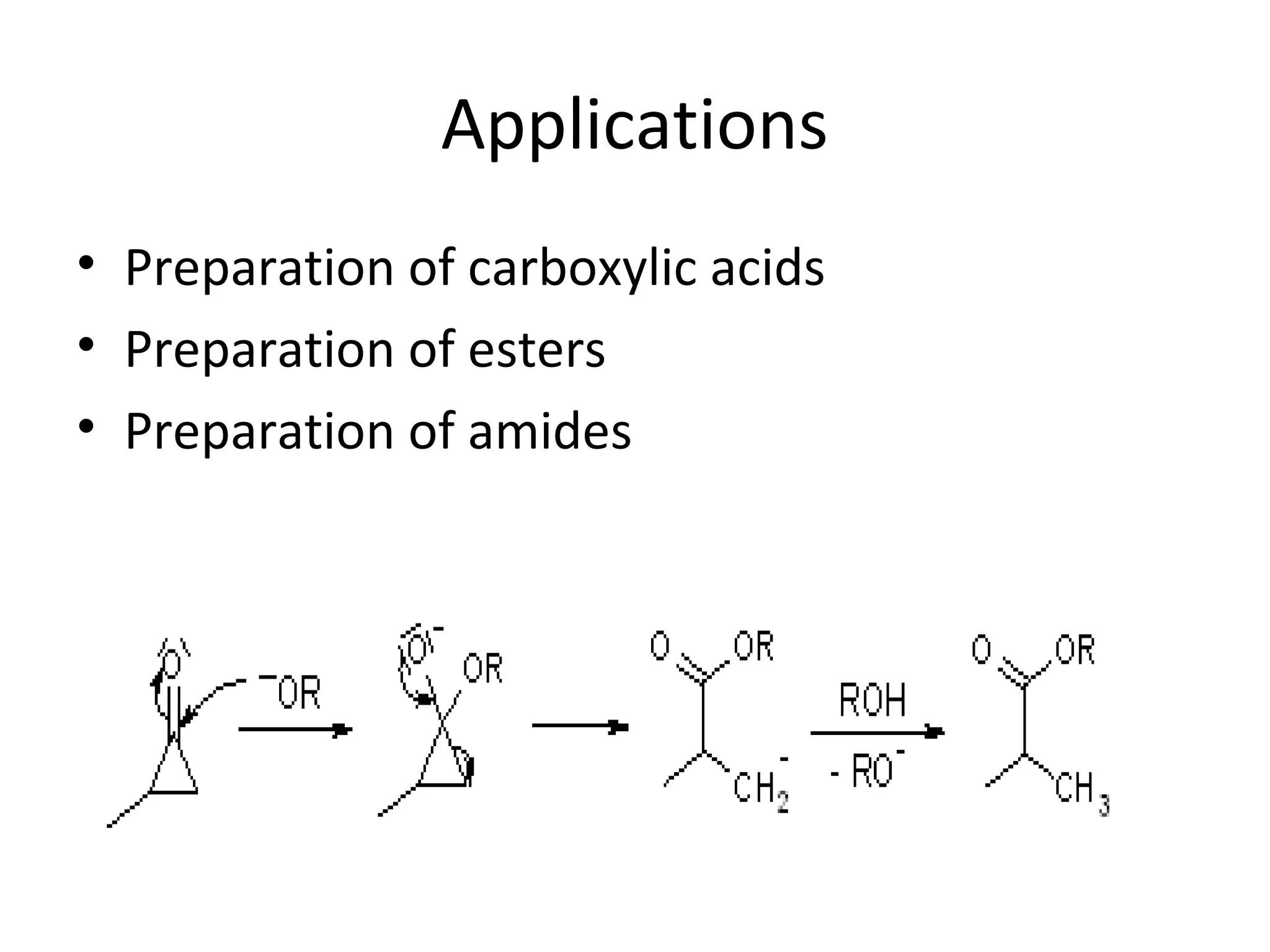
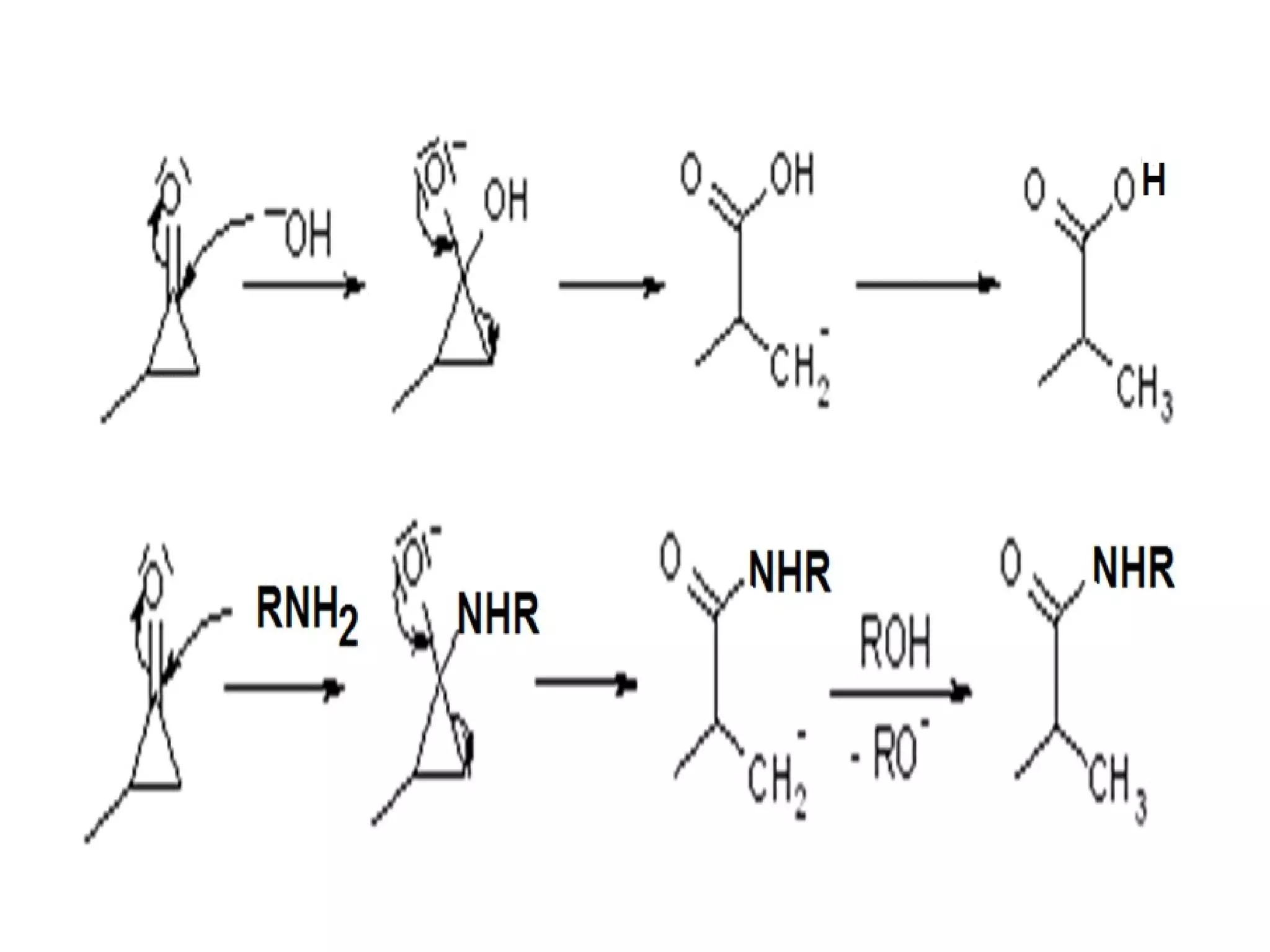
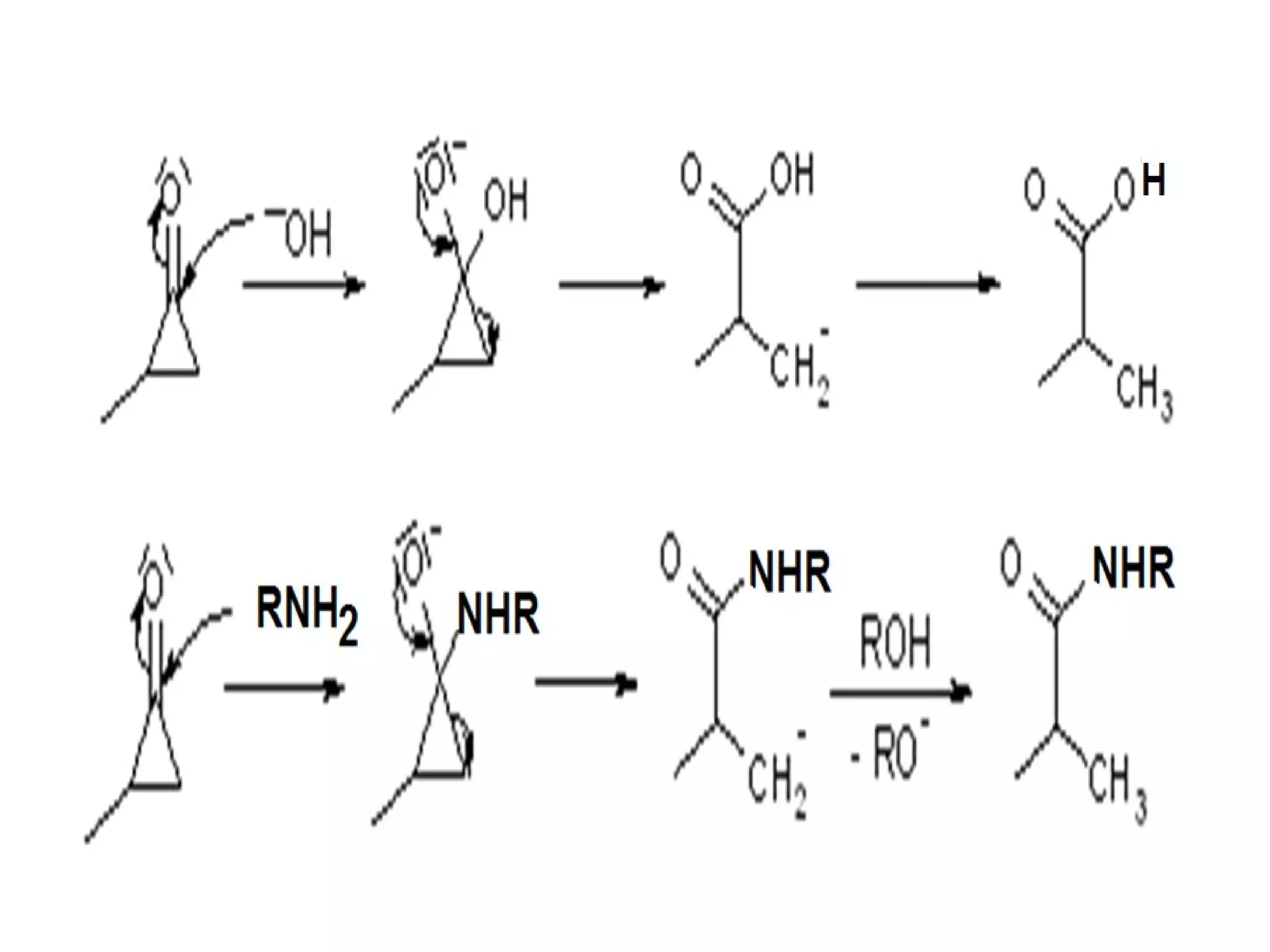

The Favorskii rearrangement is a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acids or their derivatives. It involves the formation of an enolate away from the halogen that cyclizes to a cyclopropanone intermediate, which is then attacked by a nucleophile like hydroxide or an alkoxide base to yield an acid, ester, or amide through ring contraction. The reaction is useful for preparing carboxylic acids, esters, and amides.









