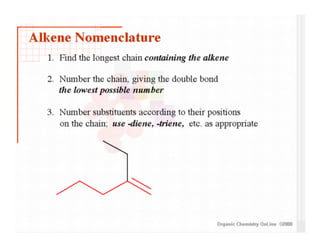
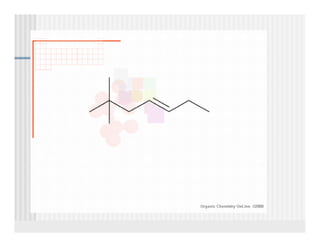
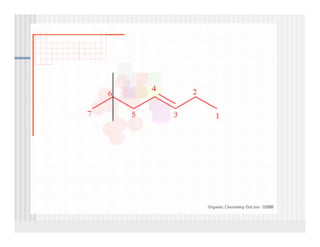
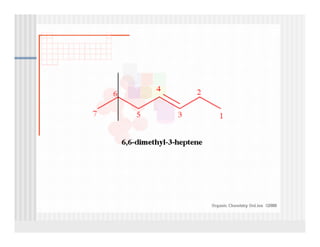
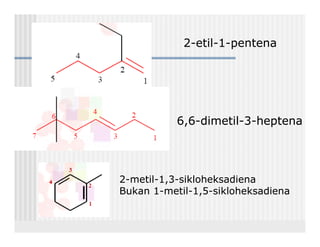
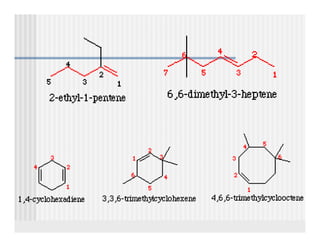
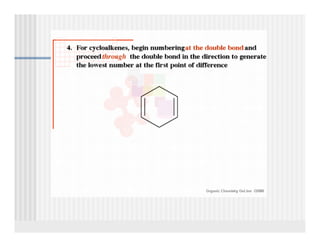
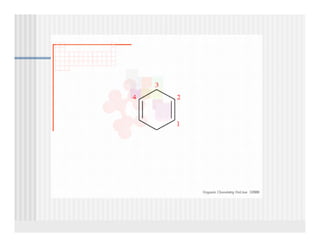
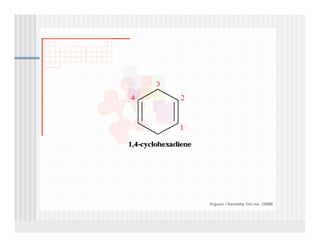
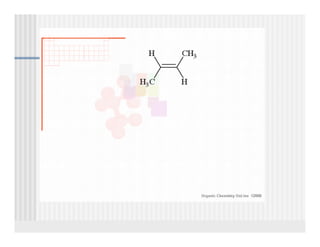
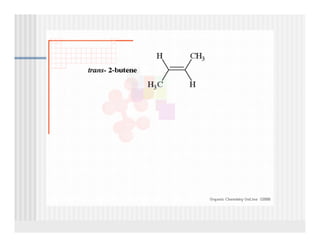
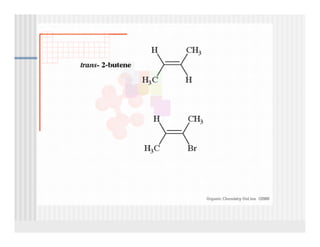
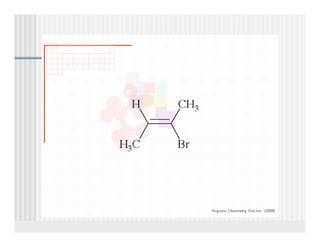
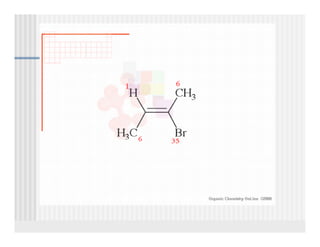
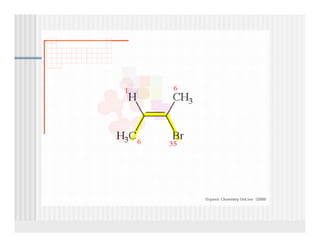
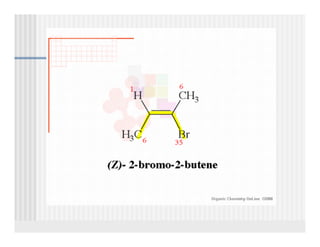
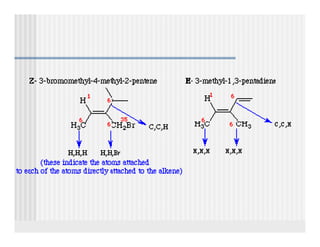
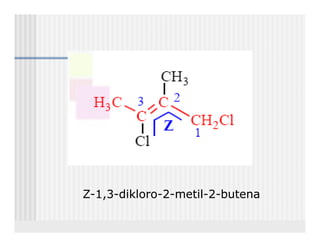
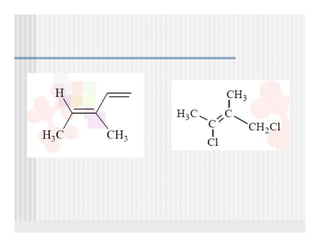
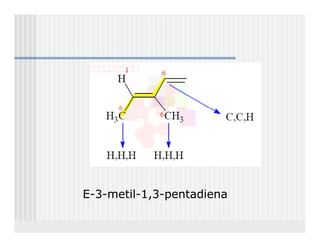
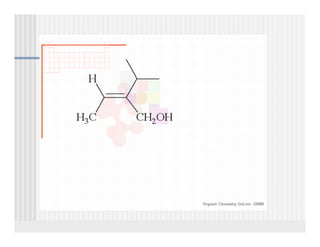
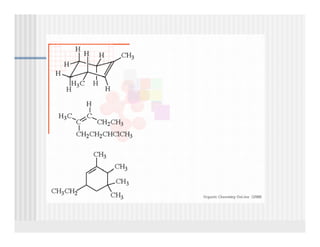
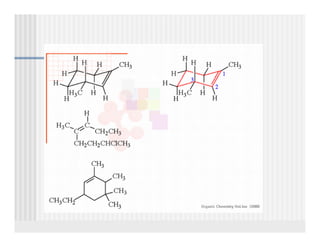
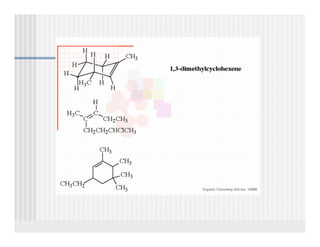
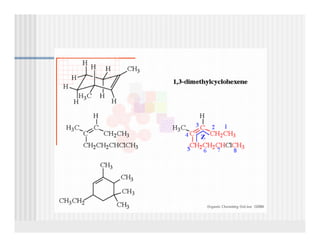
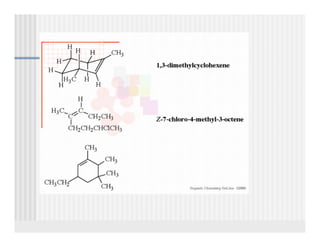
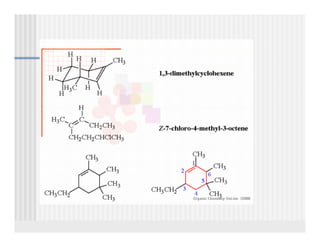
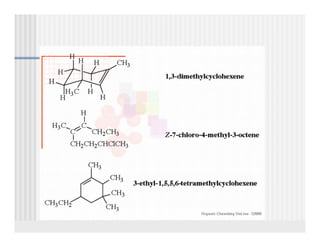
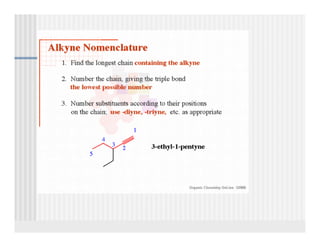
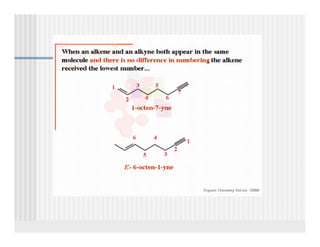
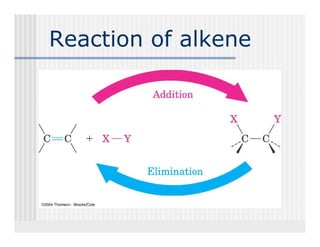
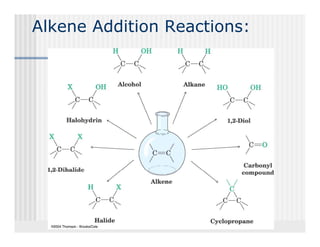
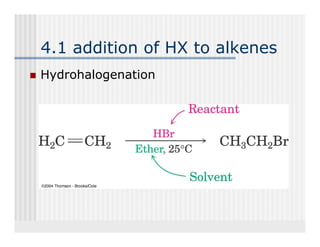
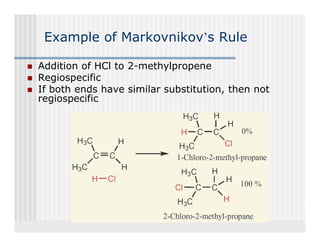
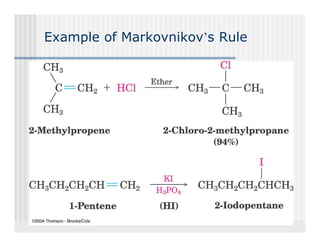
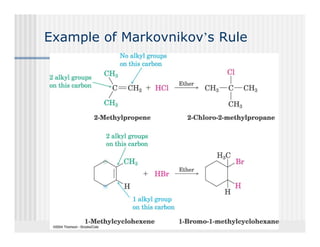
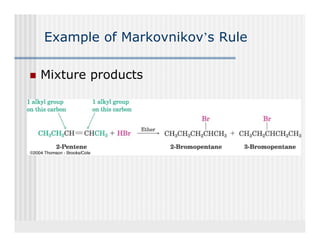
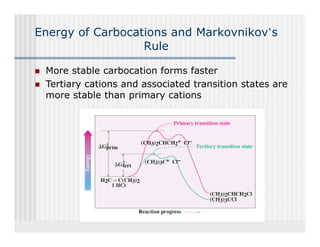
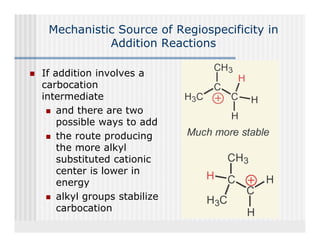
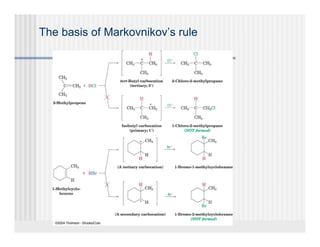
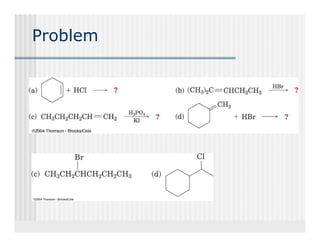
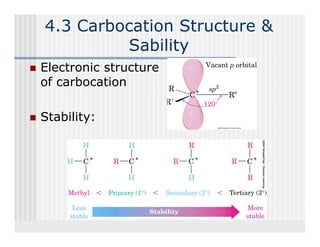
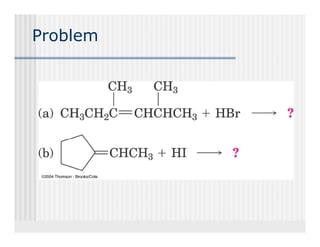
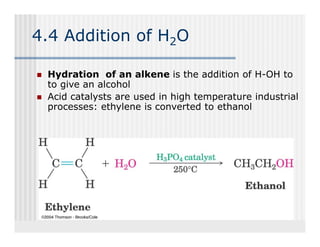
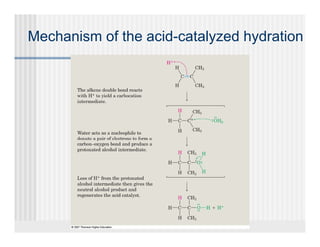
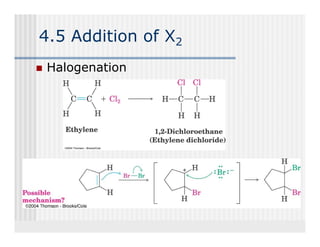
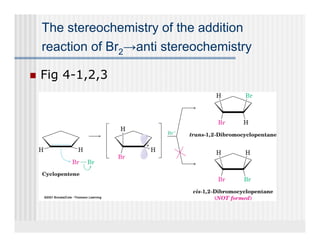
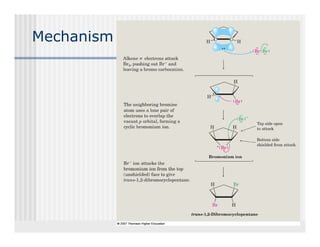
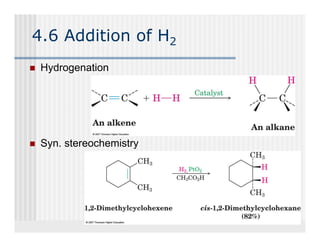
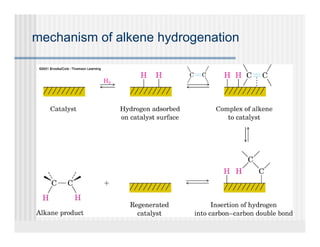
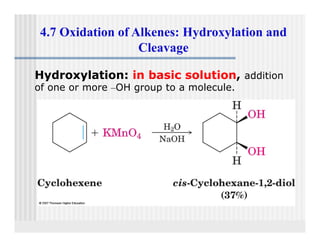
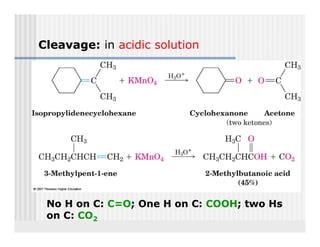
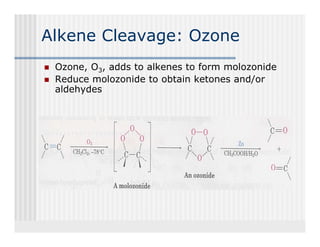
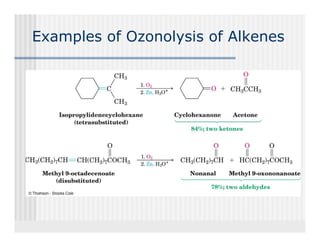
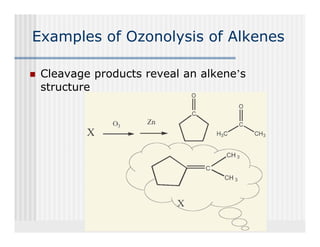
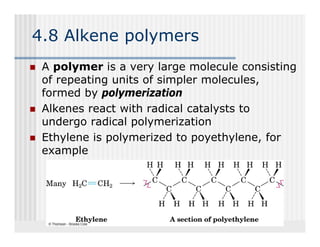
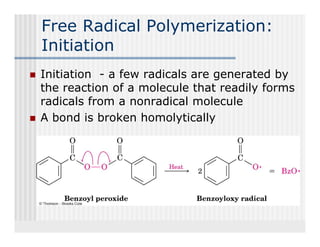
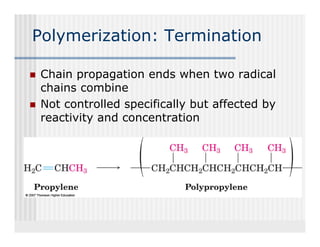
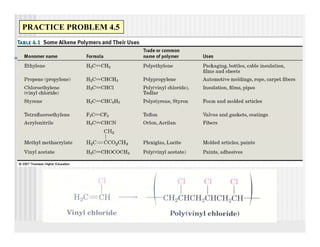
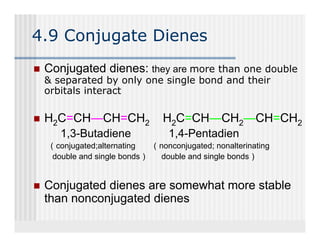
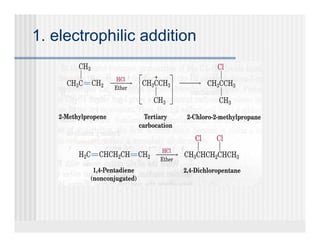
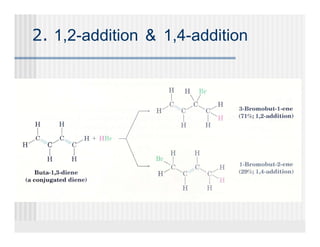
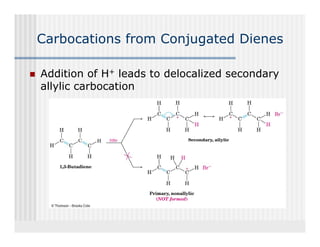
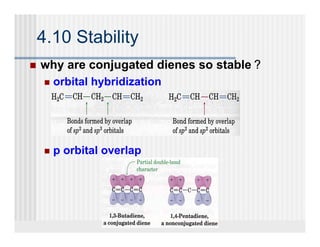
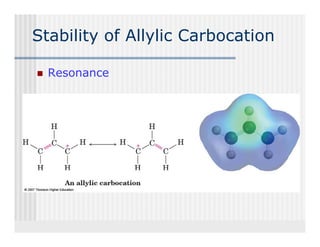
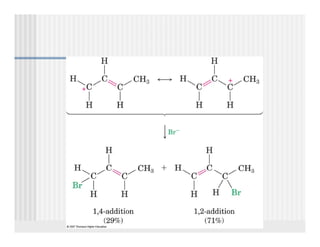
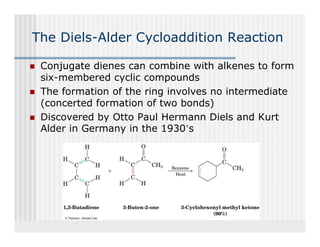
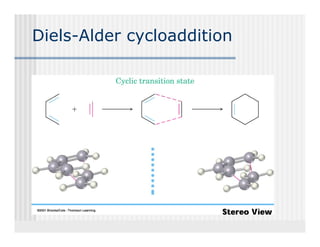
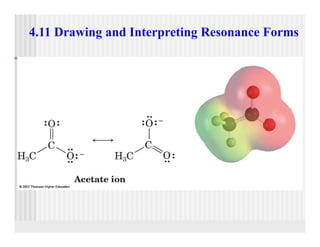
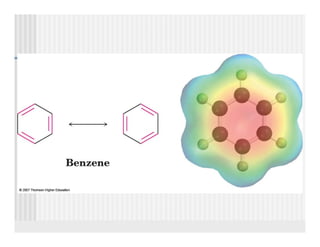
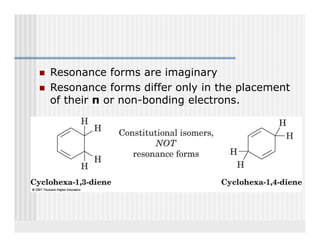
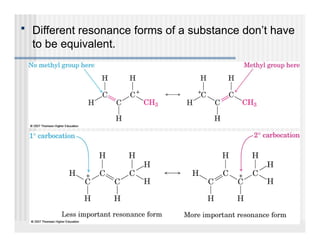
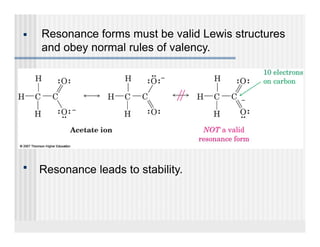
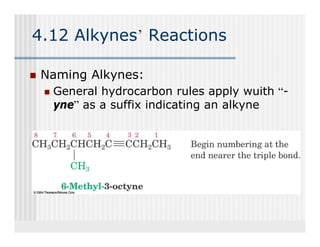
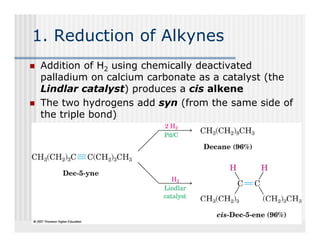
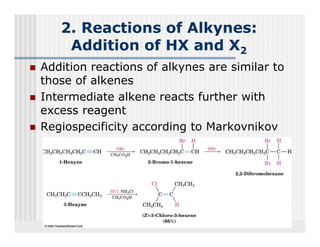
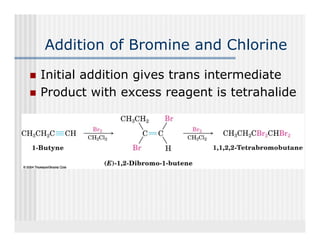
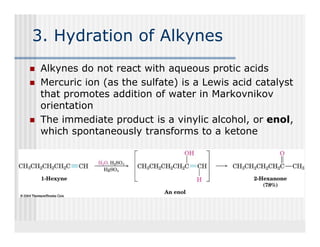
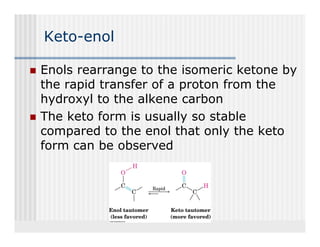
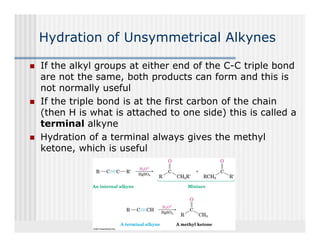
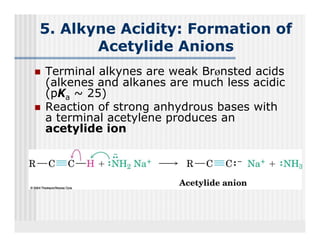
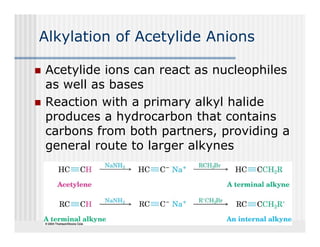
This document provides an overview of alkenes and alkynes reactions. It discusses addition reactions of alkenes including hydrohalogenation, hydration, halogenation, hydrogenation, oxidation, and polymerization. It also covers conjugated dienes, the Diels-Alder reaction, and drawing resonance forms. For alkynes, the document discusses reduction, addition reactions, hydration, oxidative cleavage, acidity, and acetylide anion formation and reactions.