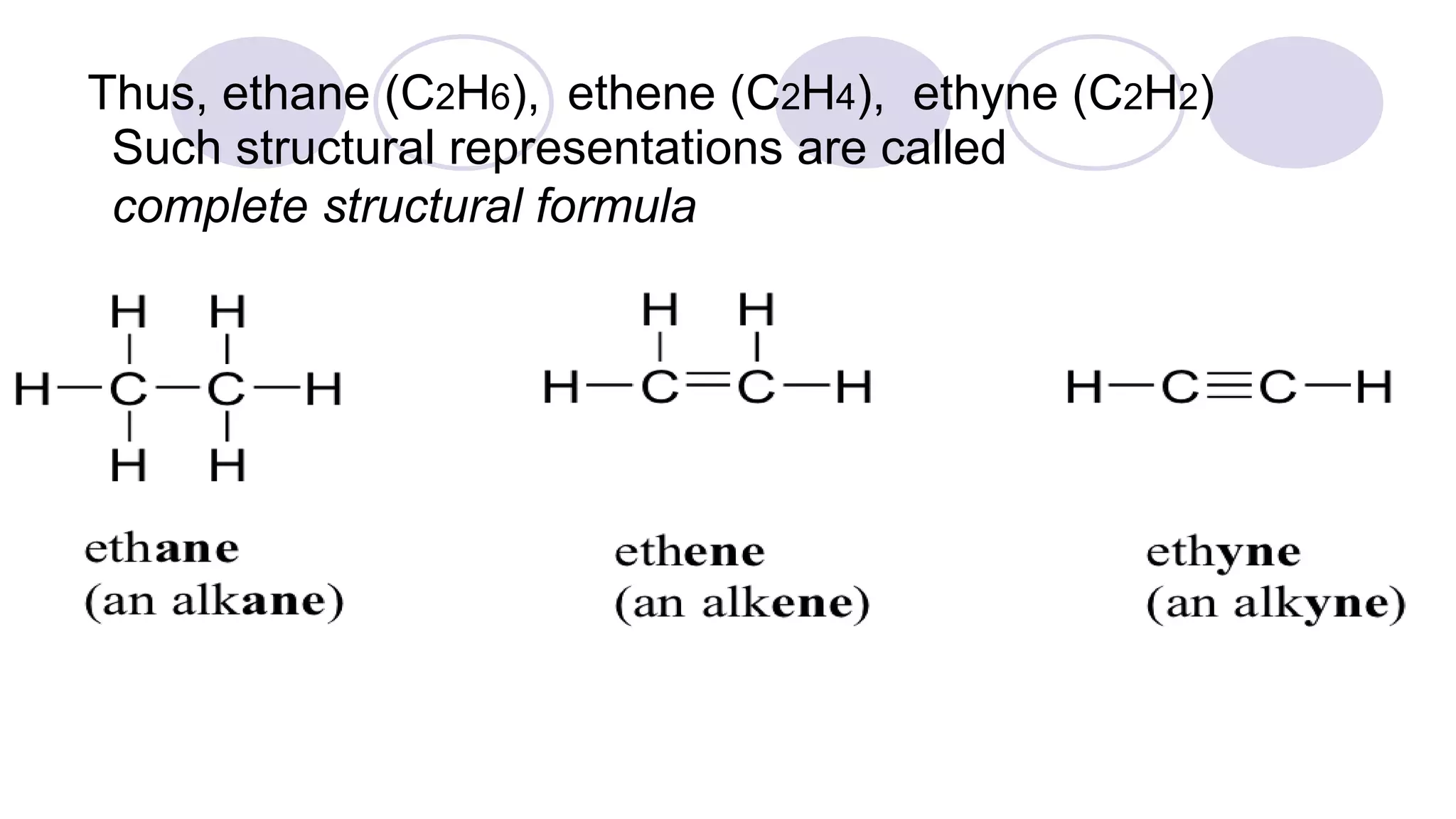
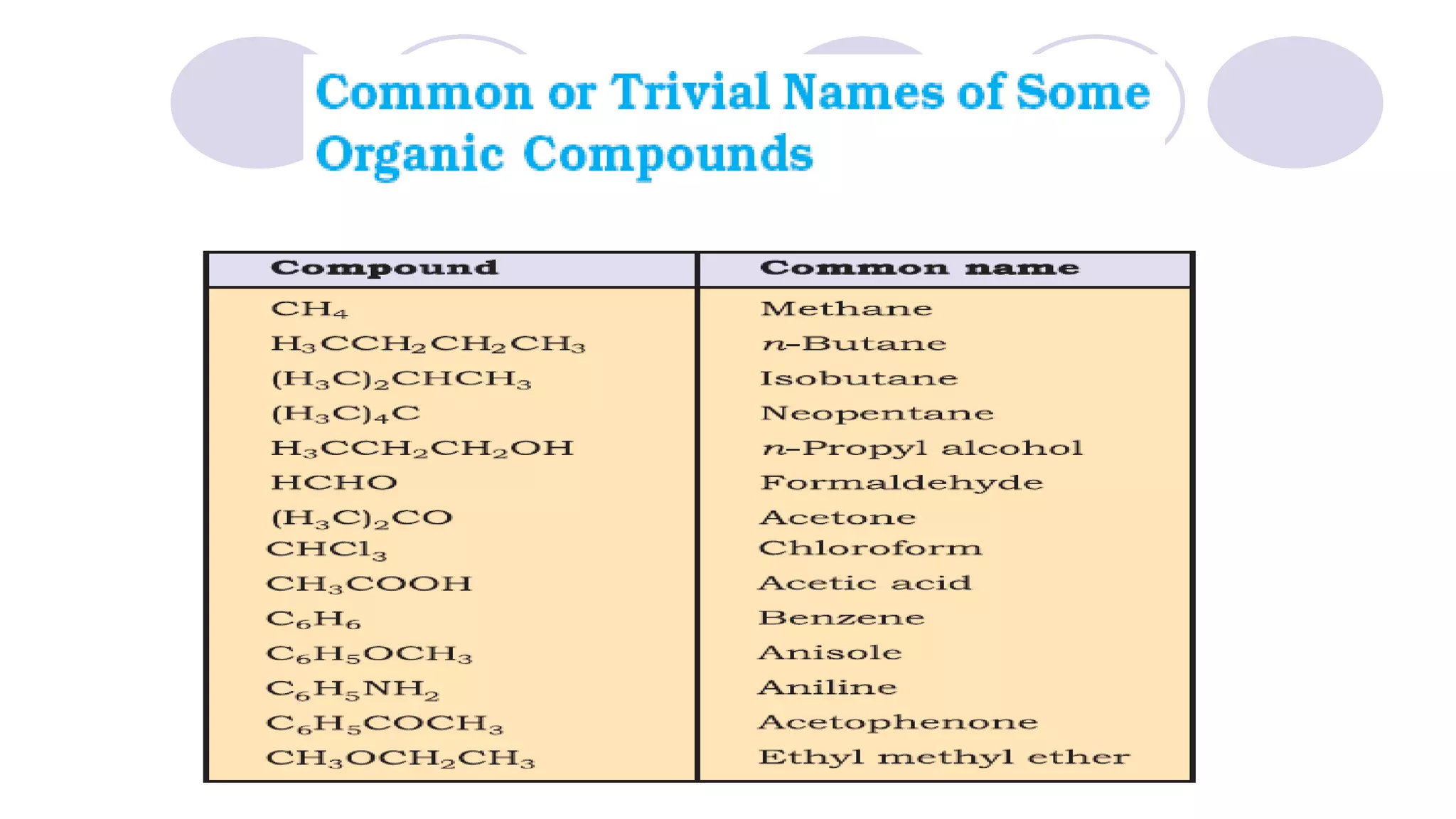
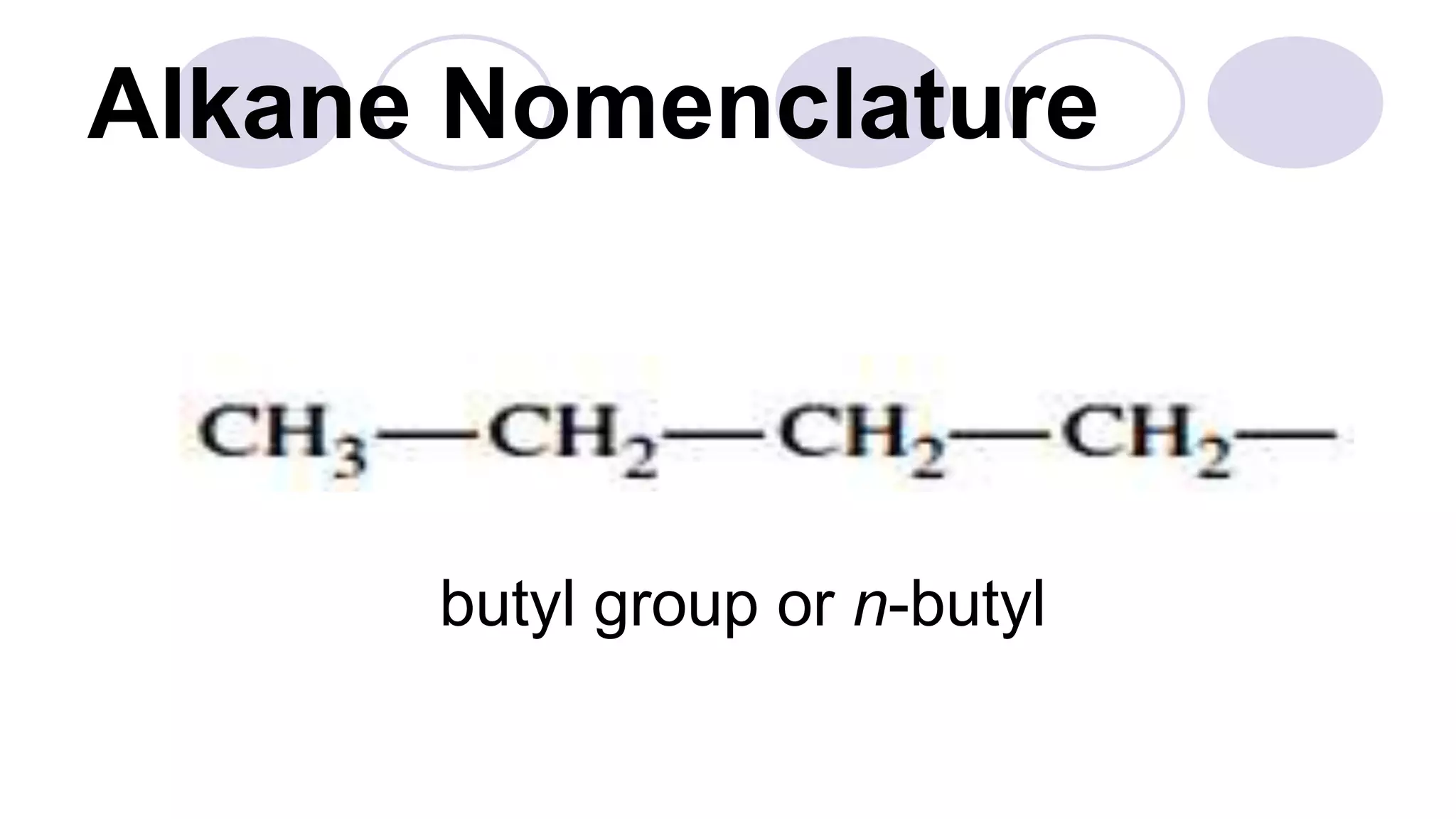
1. The document discusses various methods of representing the structures of organic compounds including Lewis structures, condensed structures, and bond line structures.
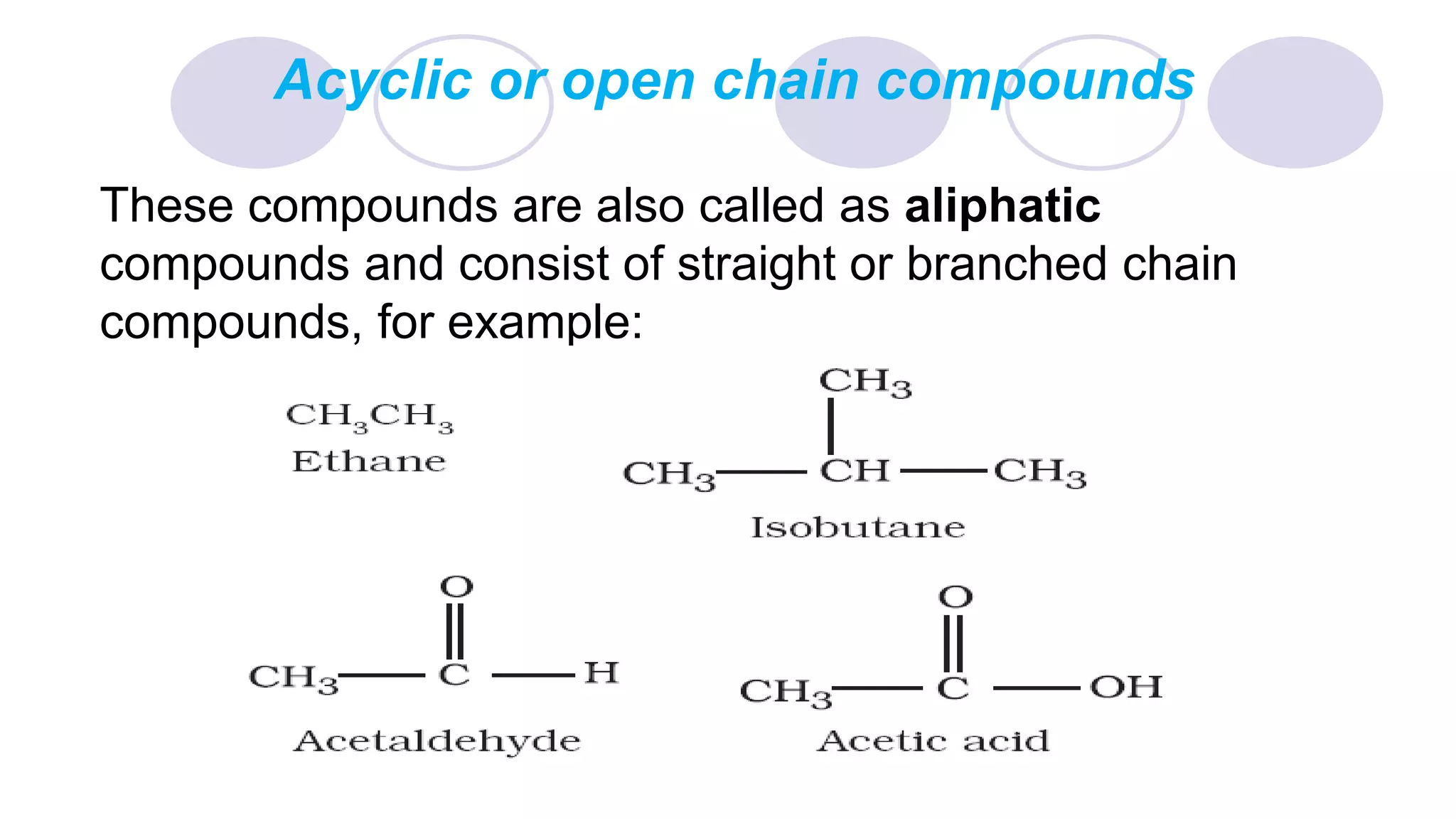
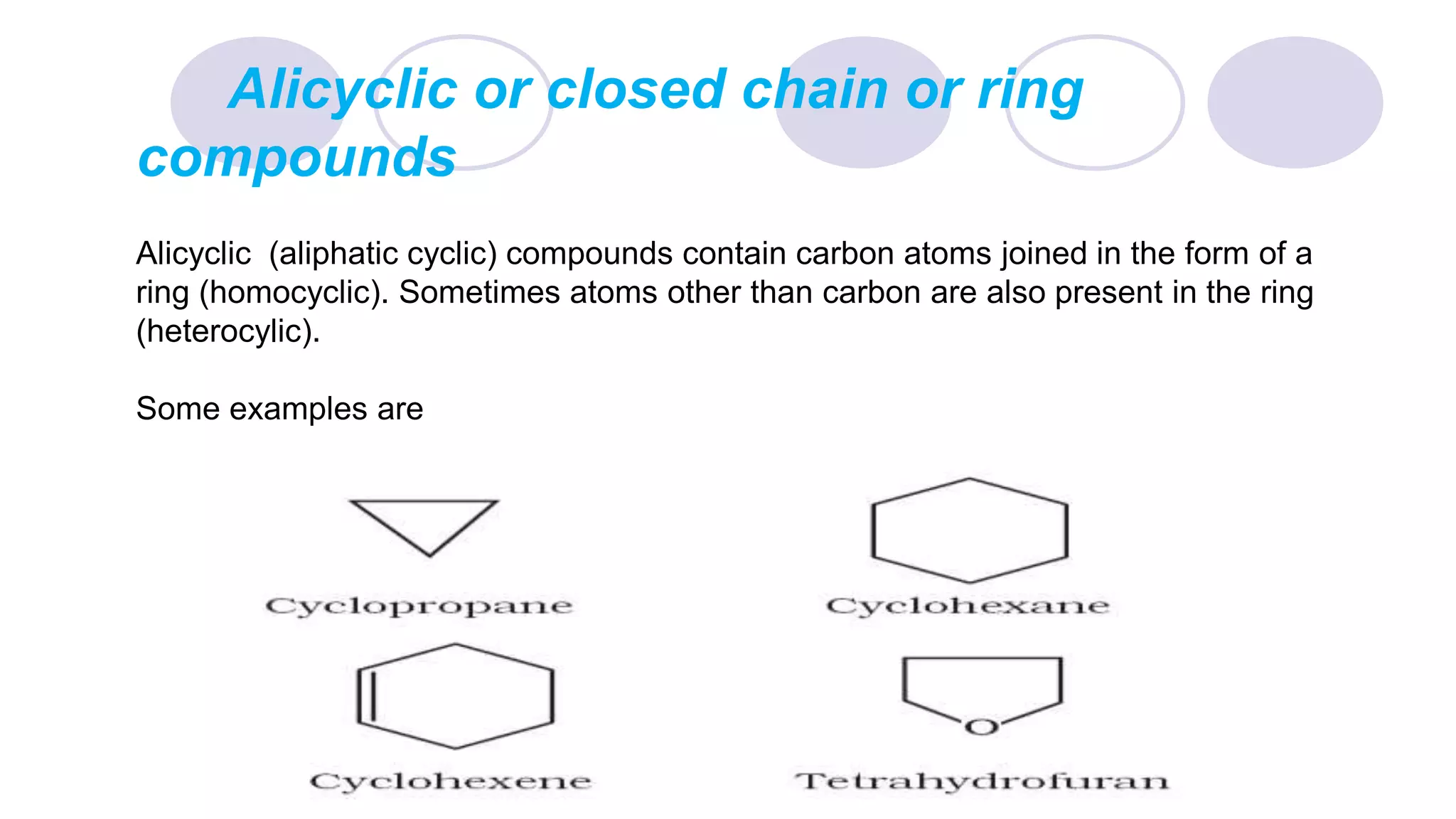
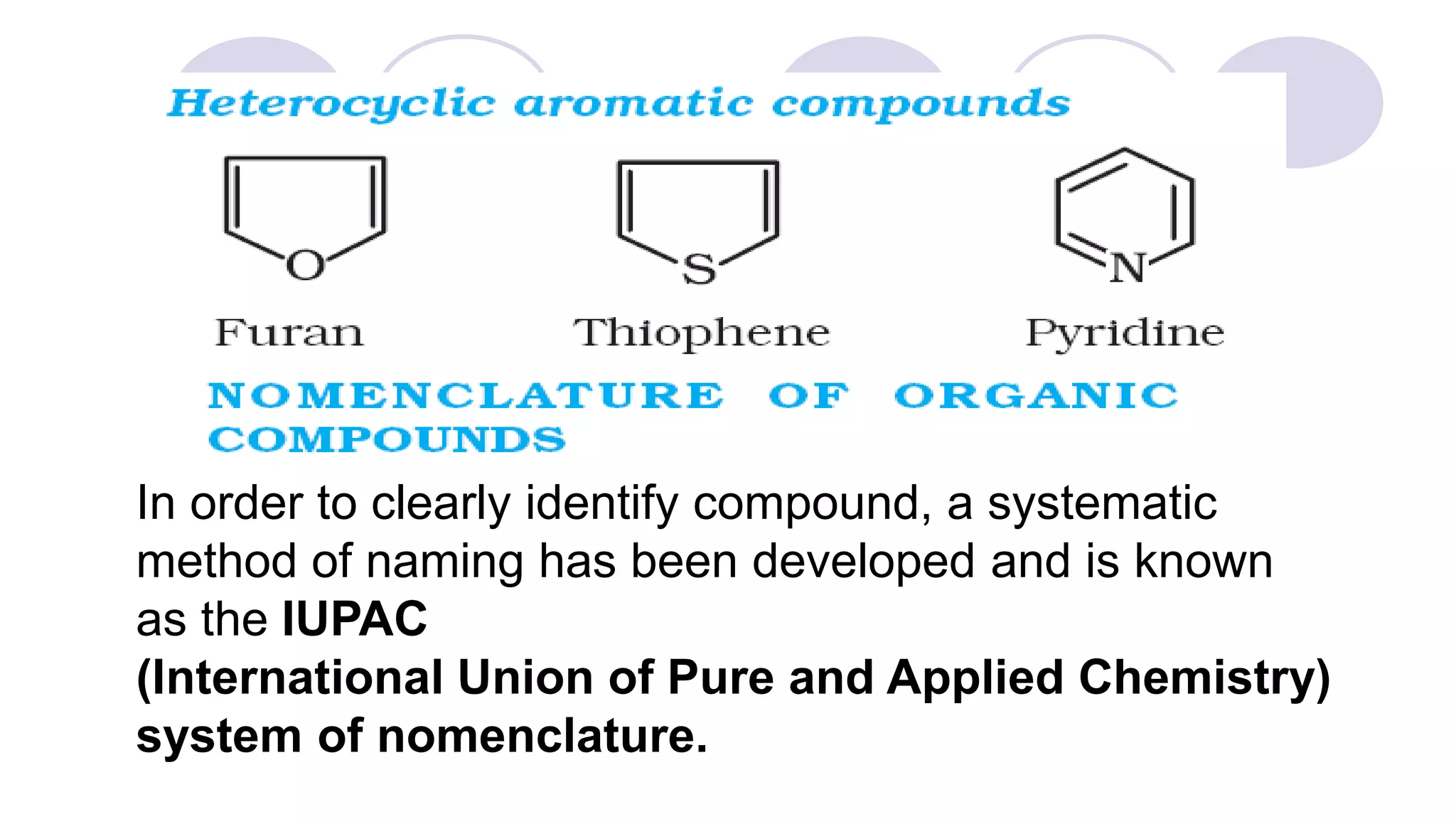
2. It also describes three main classes of organic compounds: acyclic/aliphatic compounds, alicyclic/cyclic compounds, and aromatic compounds.
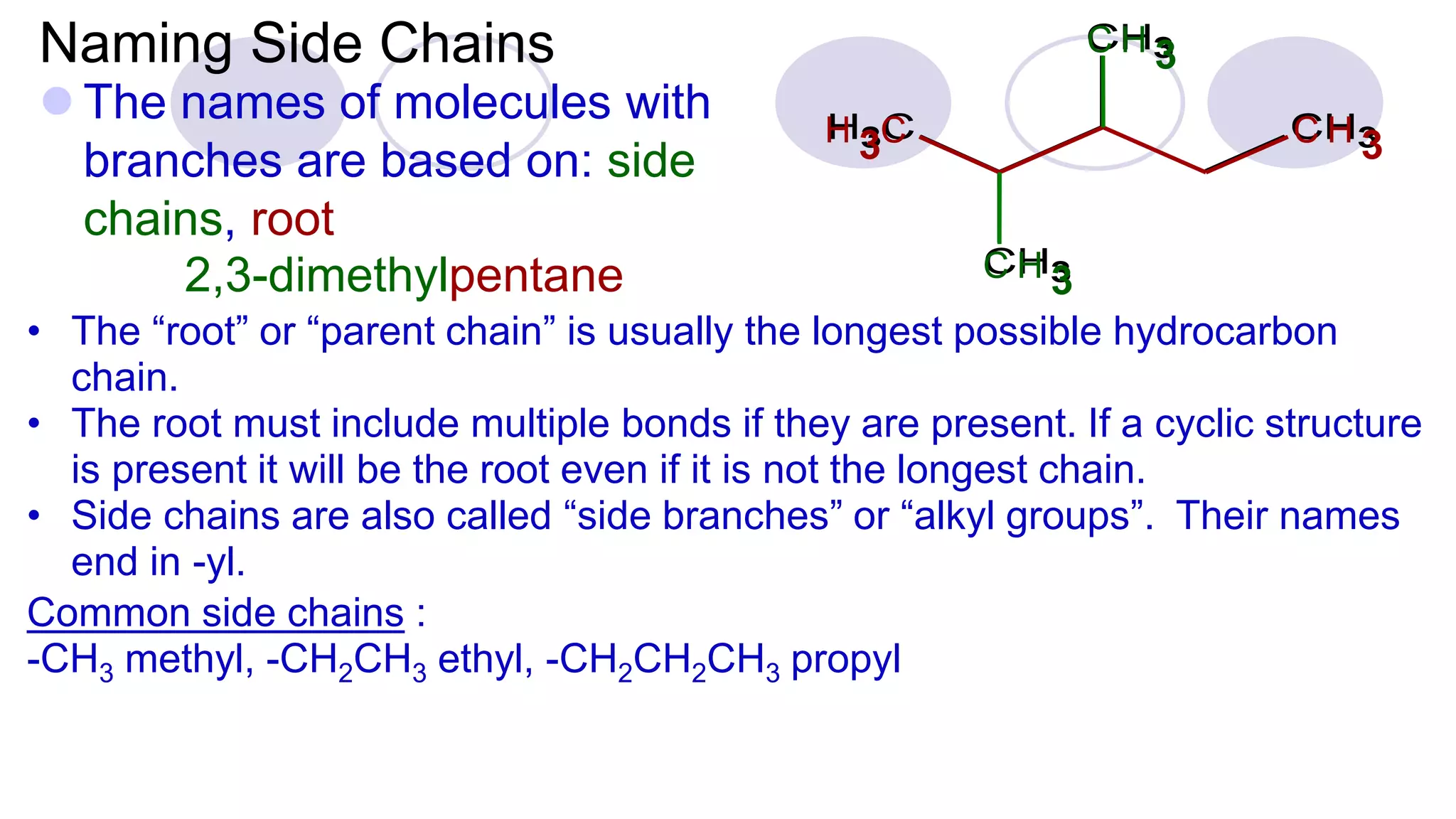
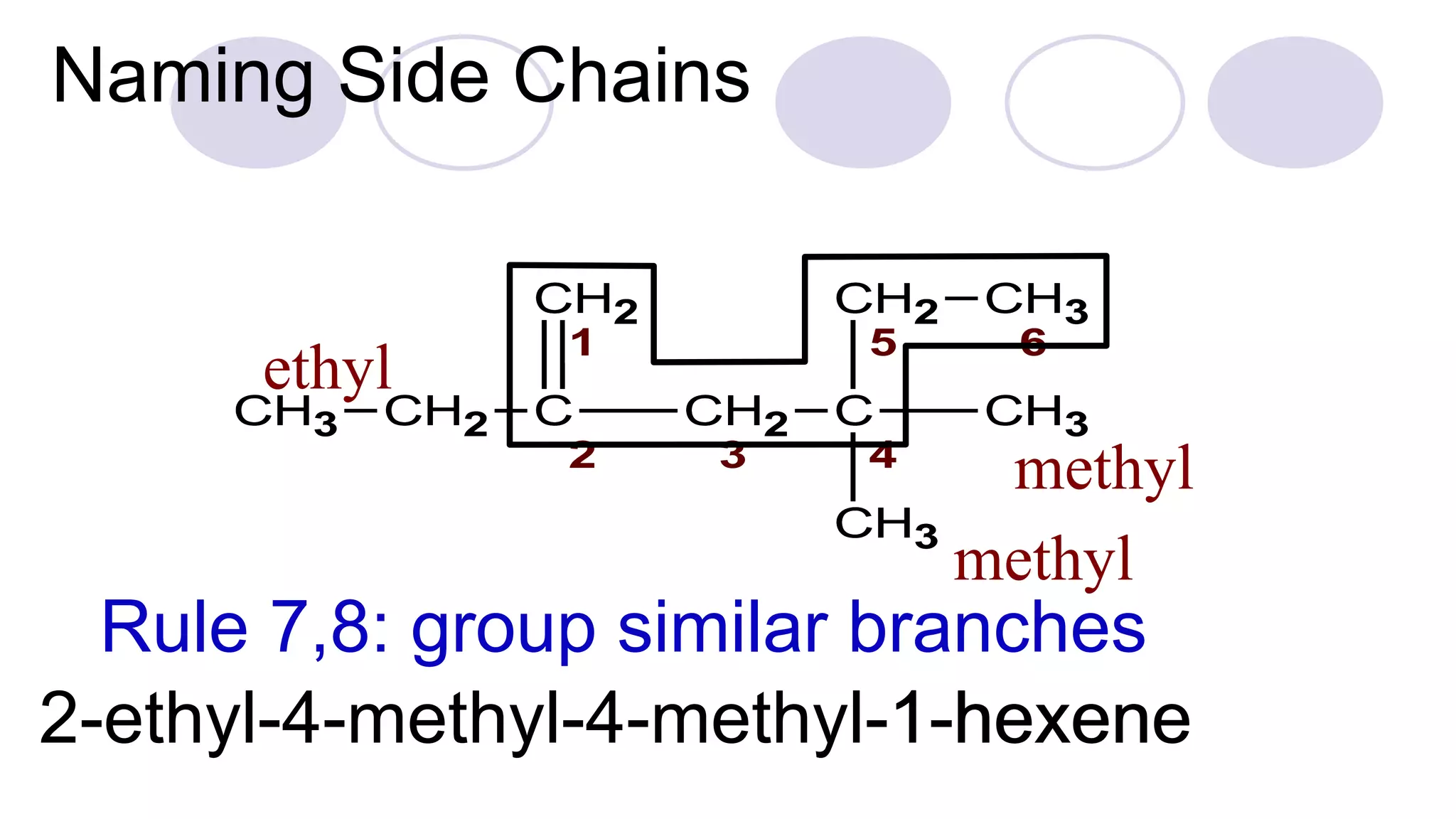
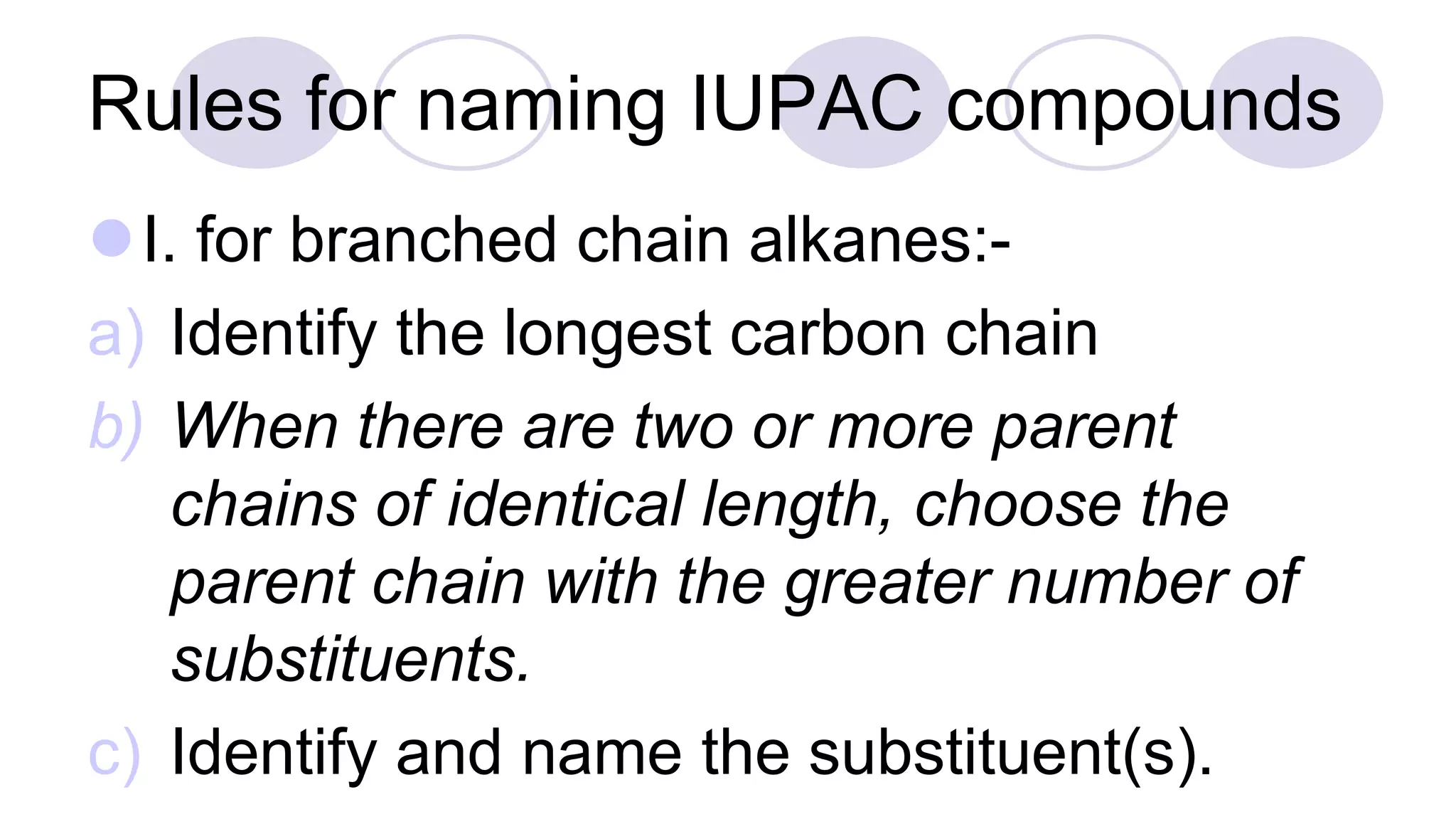
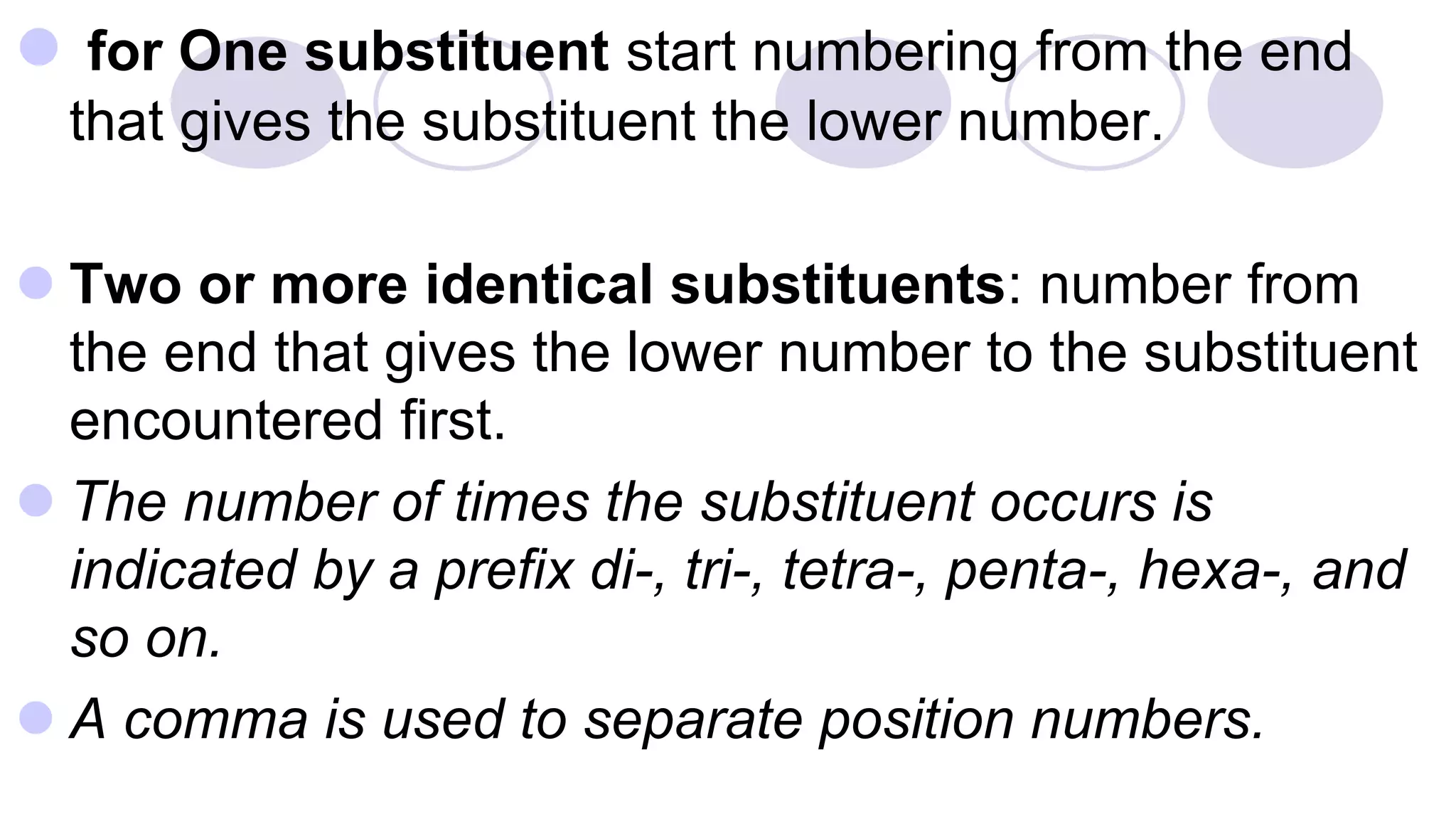
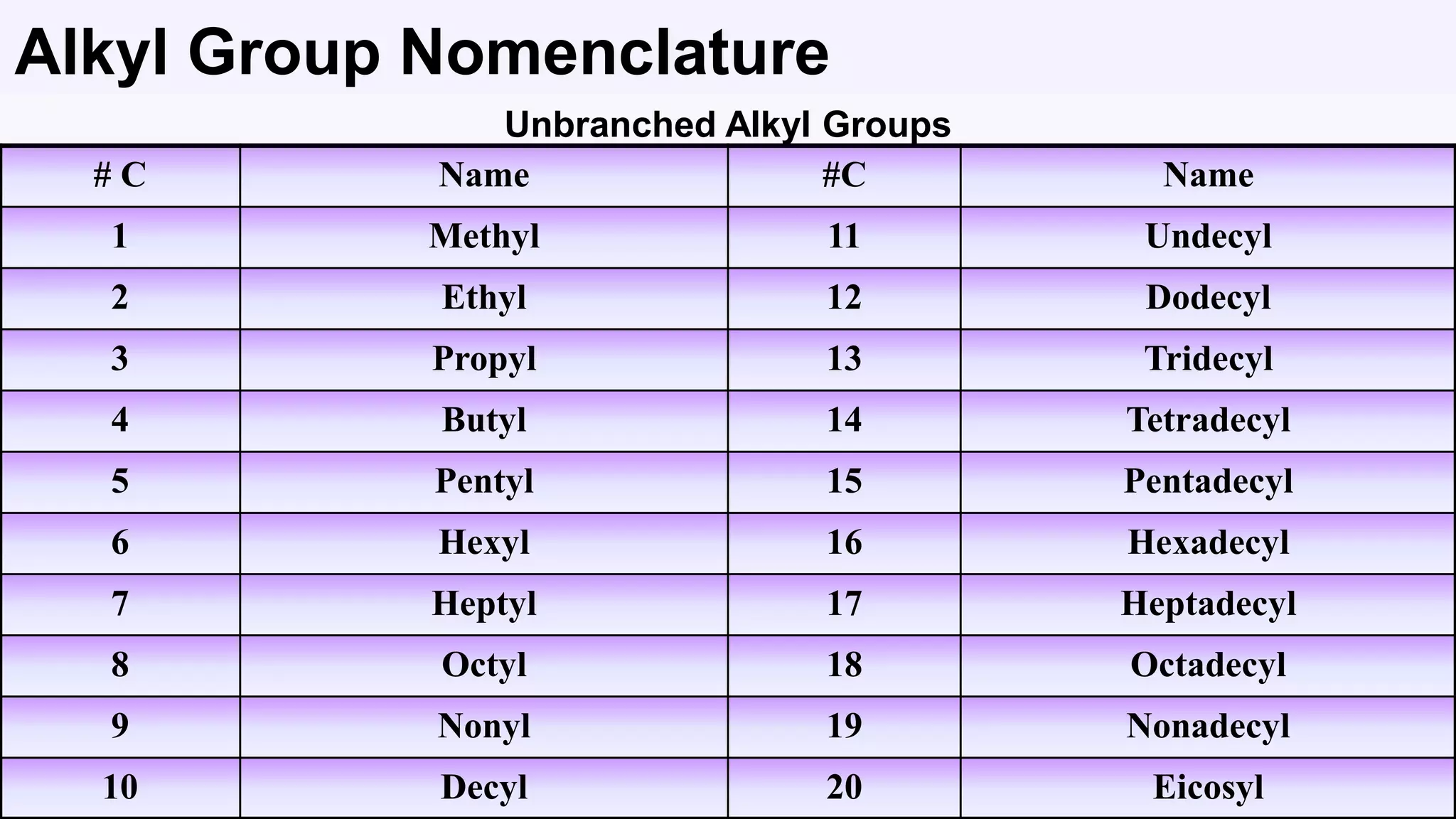
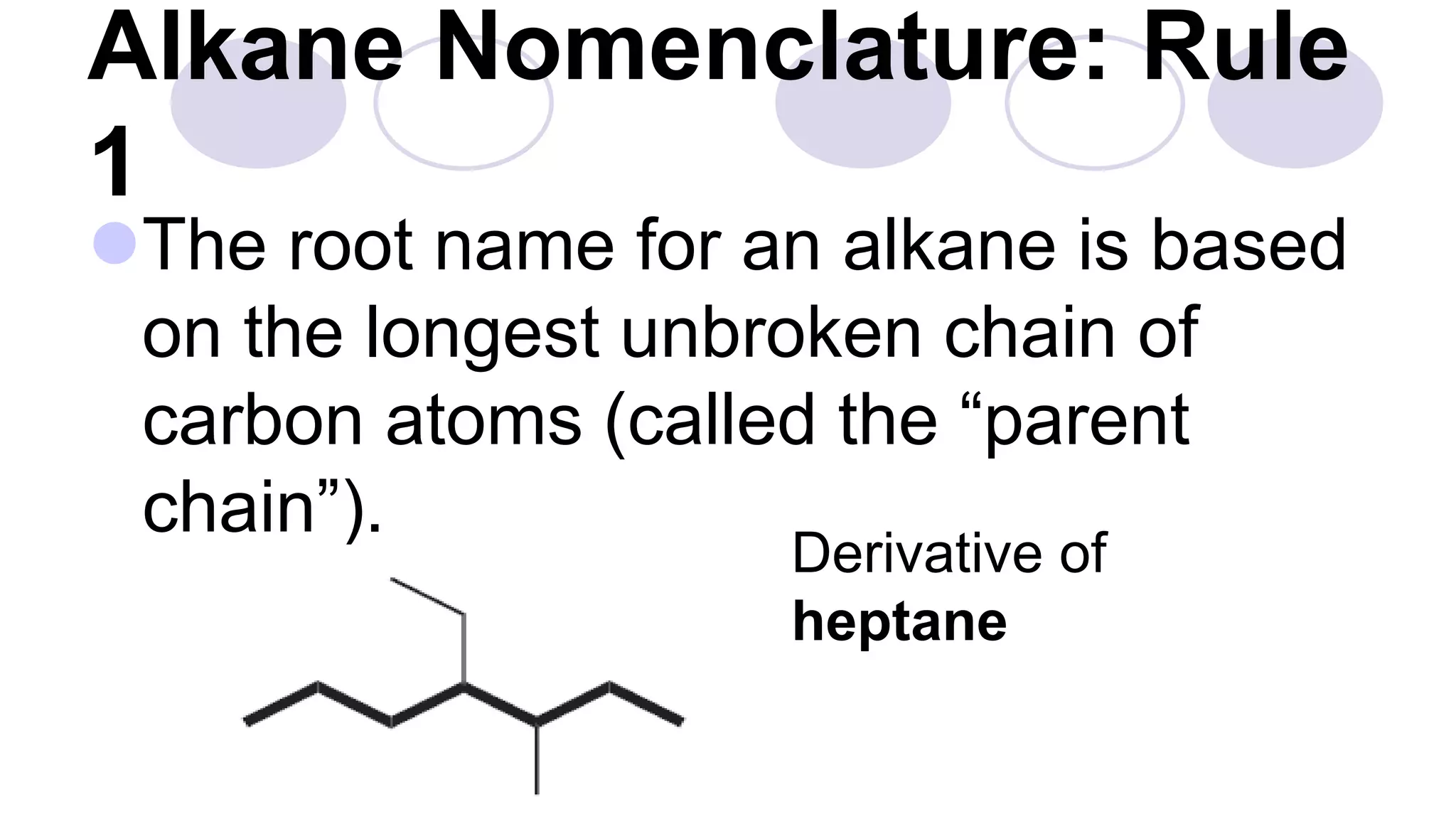
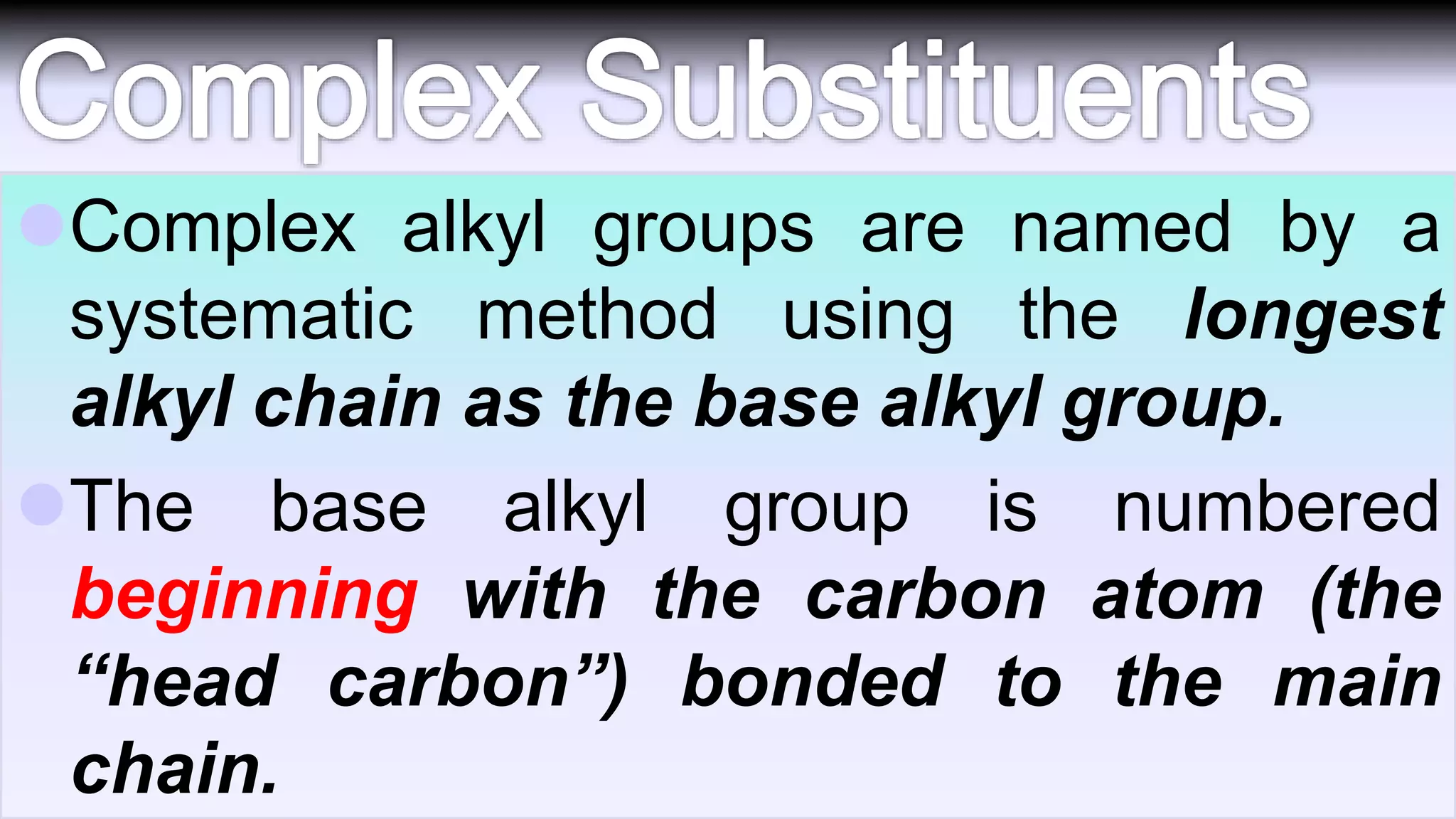
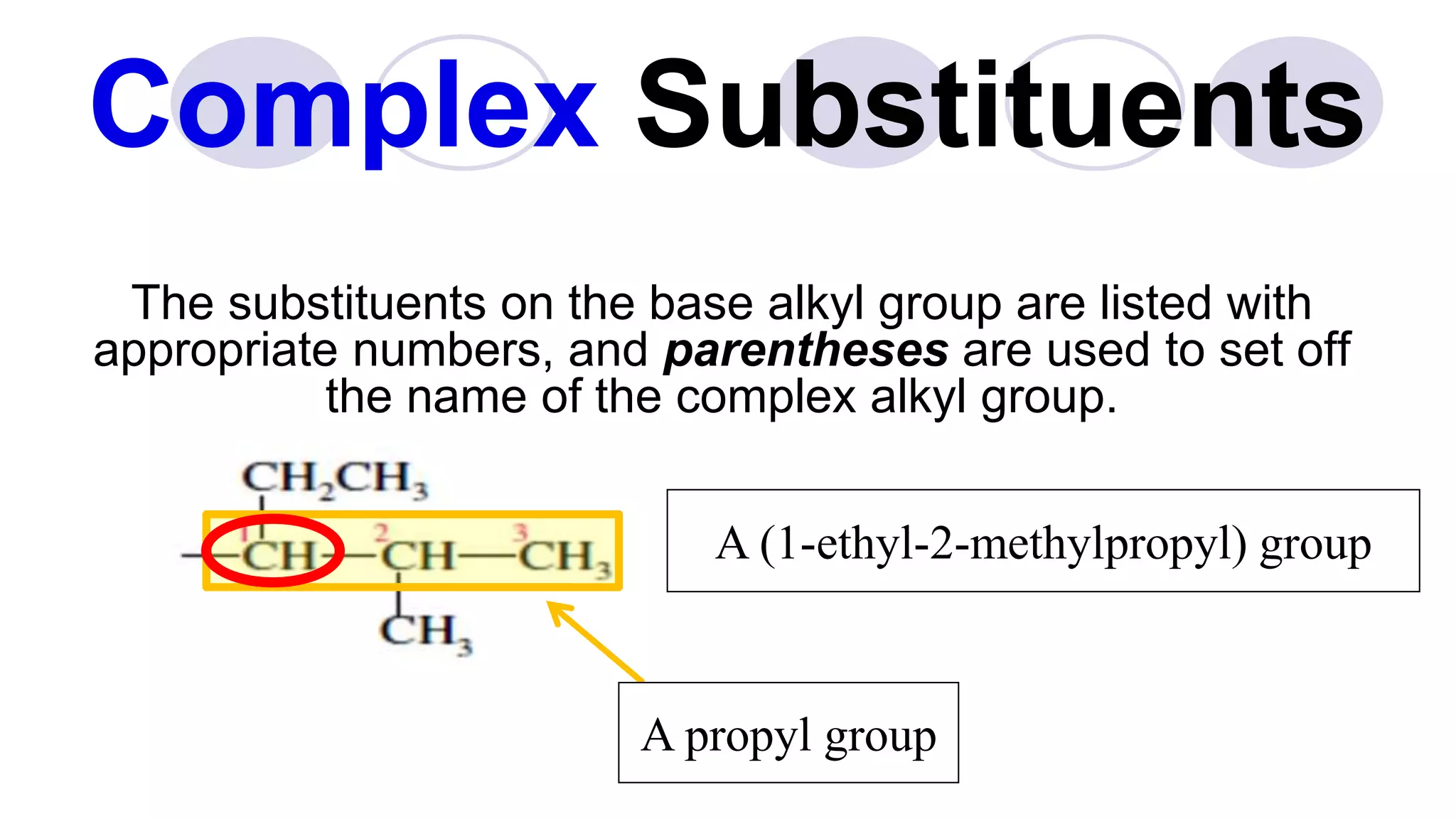
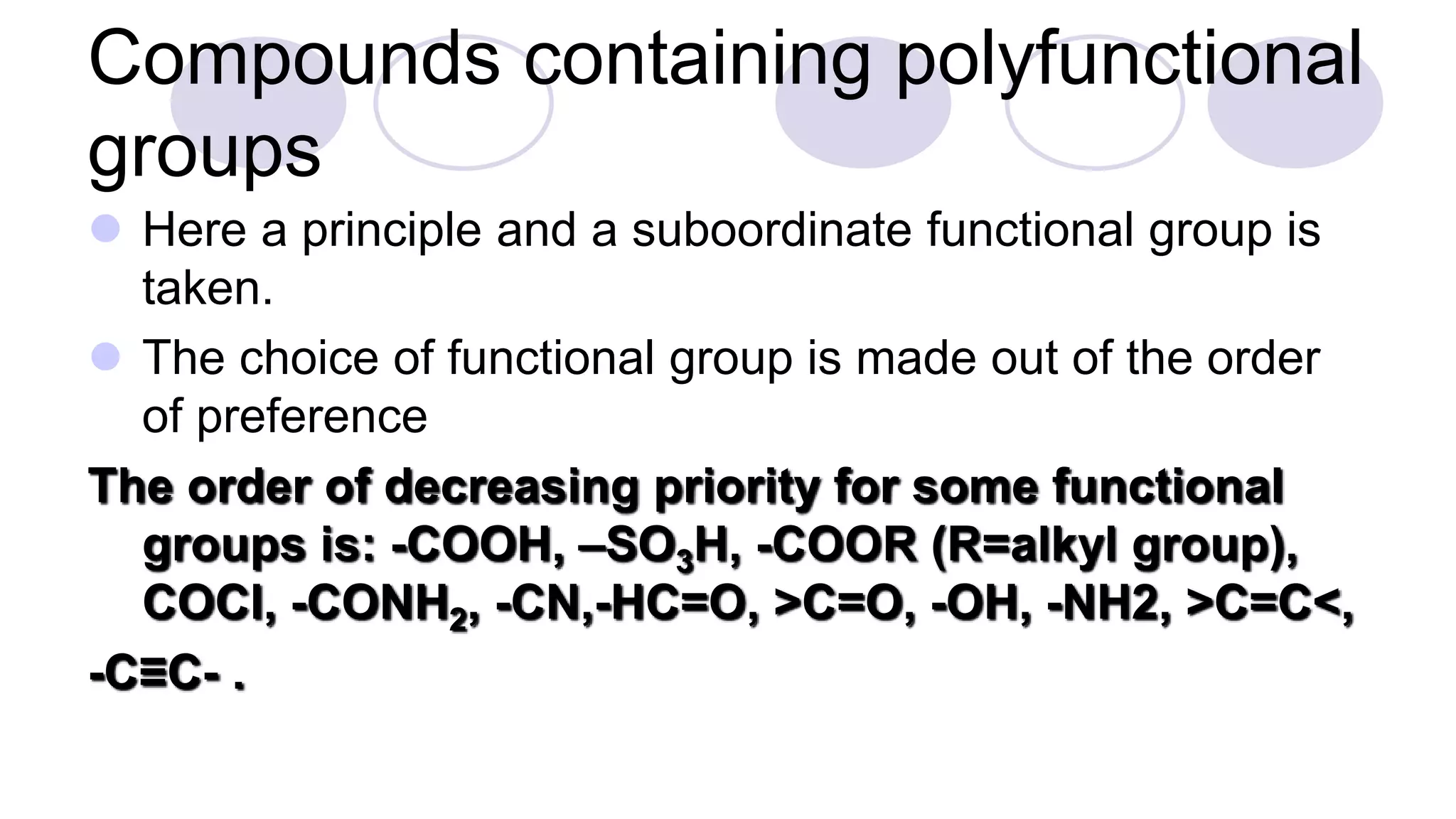
3. Rules for IUPAC nomenclature are outlined which involve identifying the parent chain, numbering carbons, indicating functional groups and branches to systematically name organic molecules.