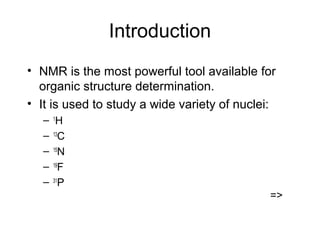
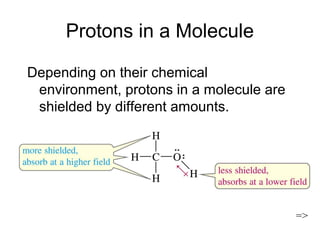
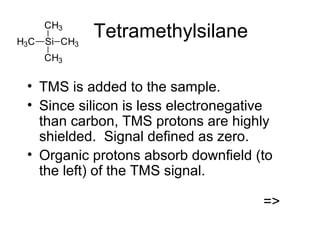
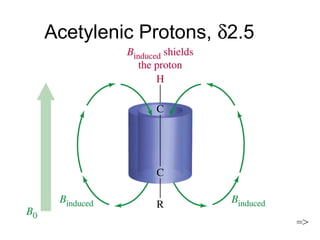
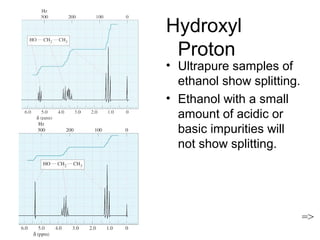
Nuclear Magnetic Resonance Spectroscopy (NMR) is a powerful tool for determining organic structures. NMR uses radio waves to analyze atomic nuclei like 1H, 13C, 15N and 19F in a magnetic field. NMR provides information on a molecule's functional groups, bonding types, and stereochemistry. Signals correspond to different nuclear environments and splitting patterns reveal molecular connectivity. Fourier transform NMR techniques and carbon-13 detection allow more detailed structural analysis. Magnetic resonance imaging (MRI) applies similar principles for noninvasive medical imaging.