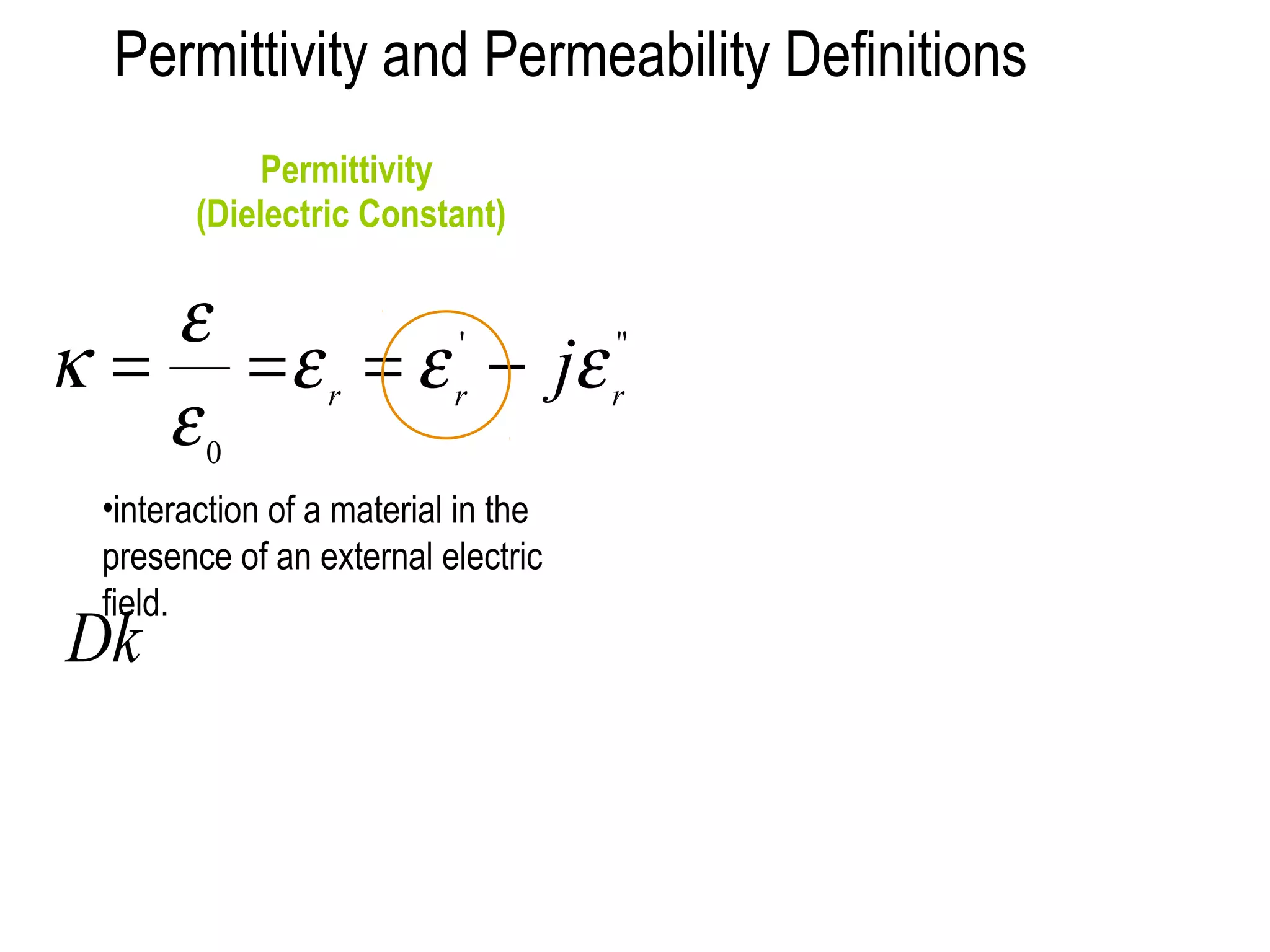
This document discusses dielectrics and their properties. It introduces dielectrics as materials that can store electric charge and energy with minimal heat loss. The document discusses how a capacitor's capacitance depends on the dielectric material between its plates, including the dielectric constant which measures a material's ability to concentrate electrostatic lines of flux. It also examines polarization in insulators when an electric field is applied and defines related terms like permittivity, dielectric constant, and loss tangent.