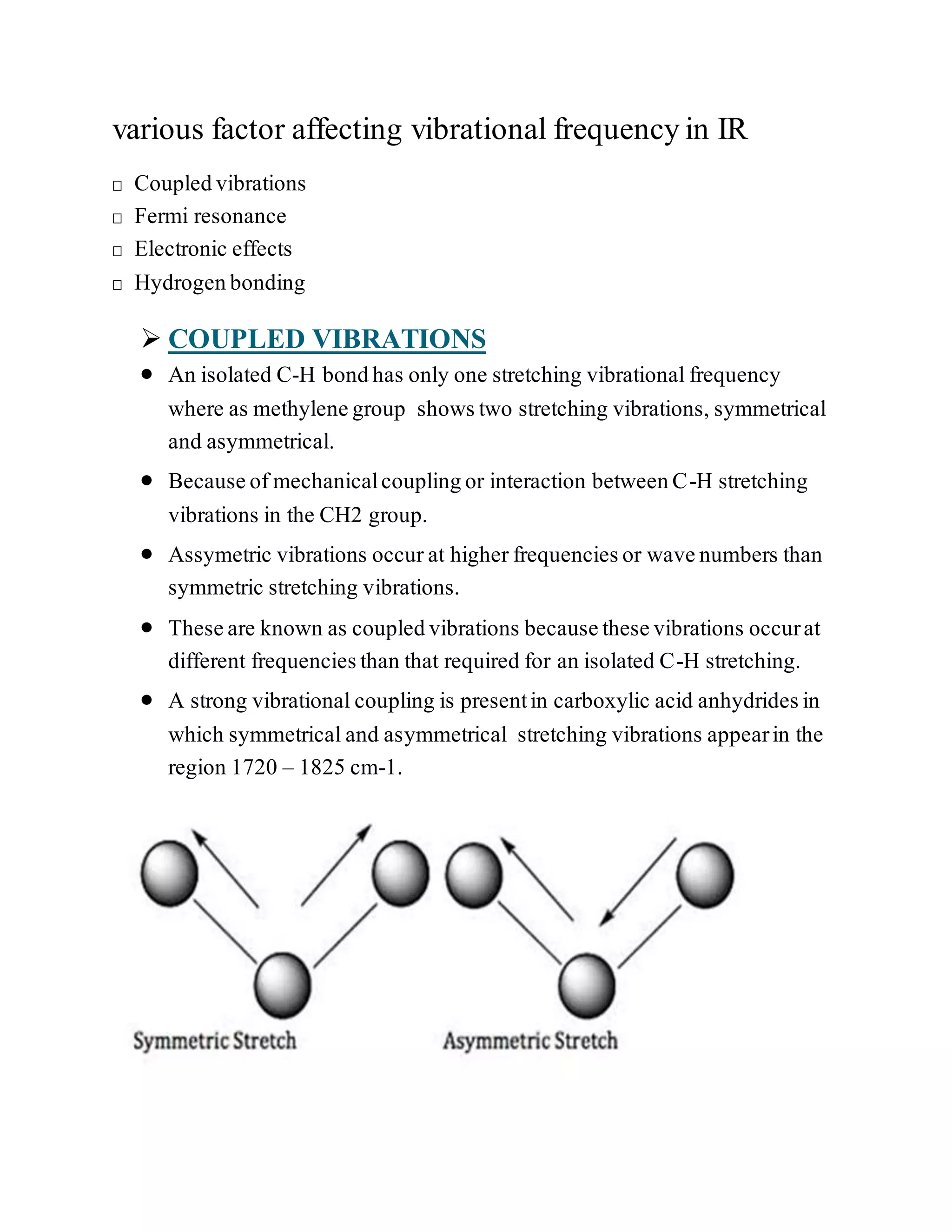
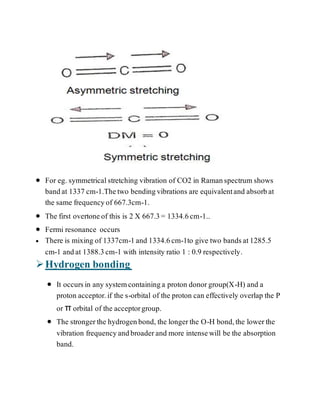
The document discusses various factors affecting vibrational frequencies in molecular vibrations, such as coupled vibrations, Fermi resonance, hydrogen bonding, and electronic effects. It explains how mechanical coupling alters C-H stretching vibrations and how interactions between different modes of vibration can create new frequencies. Additionally, it describes how hydrogen bonding intensity and electronic effects from substituents in proximity to a specific group can influence absorption frequencies.