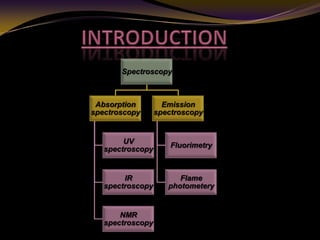
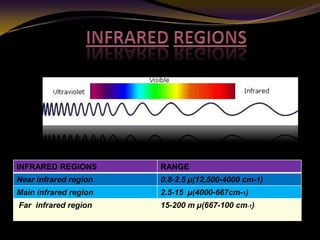
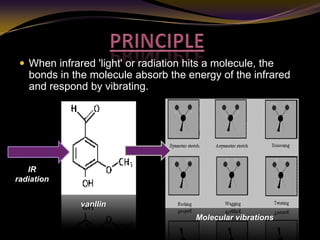
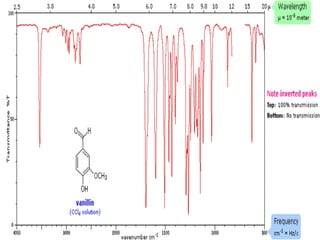
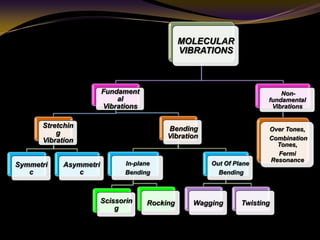
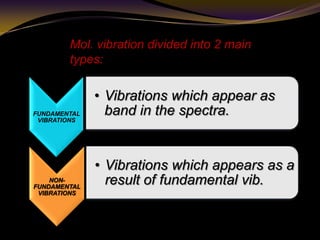
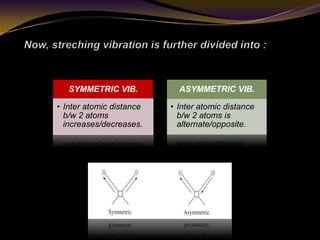
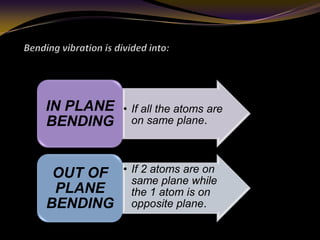
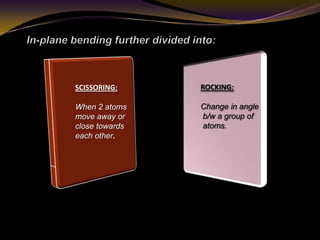
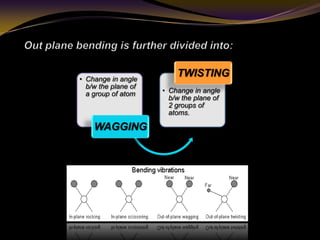
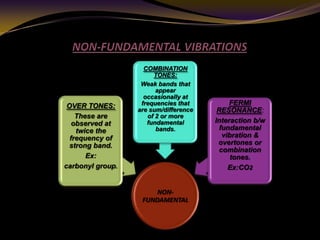
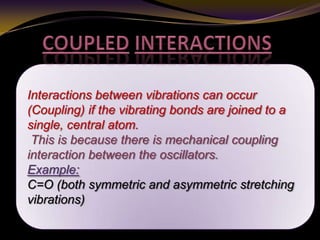
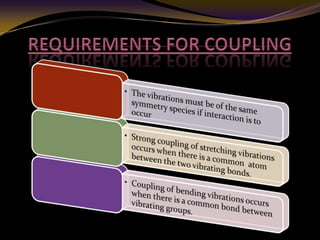
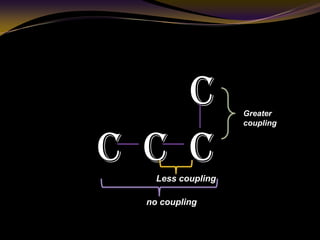
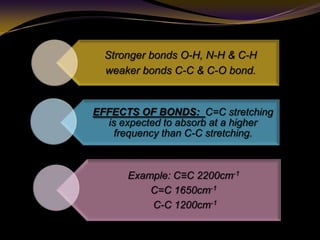
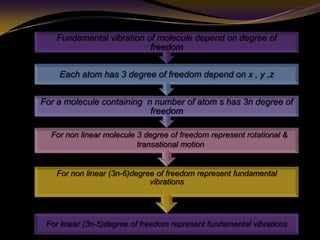
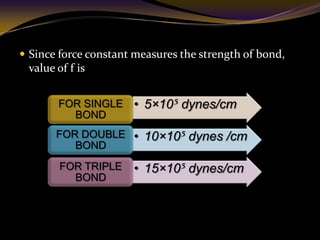
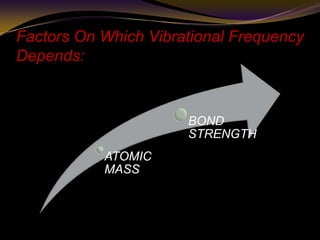
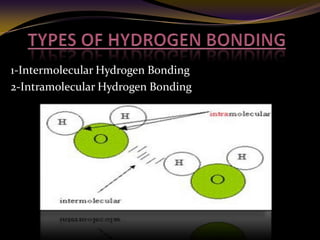
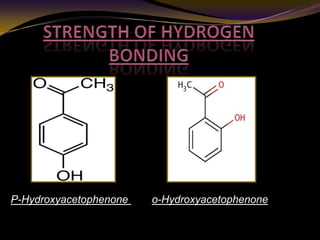
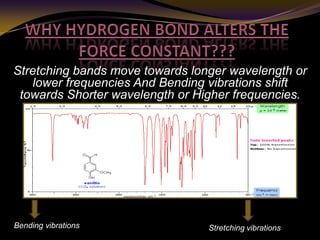
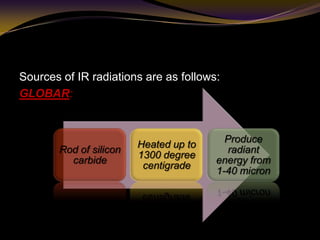
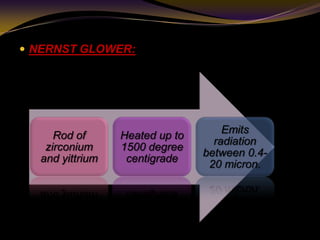
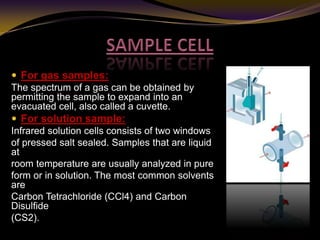
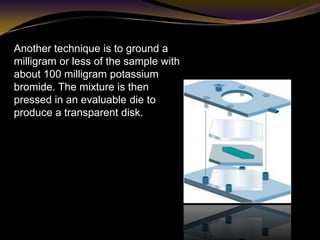
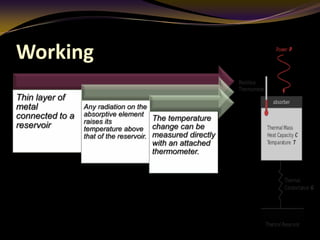
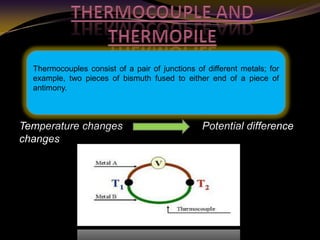
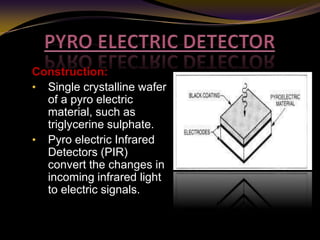
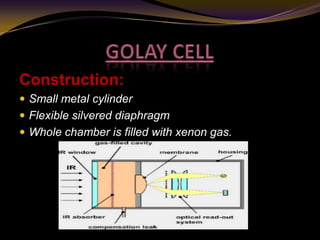
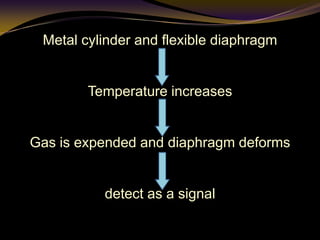
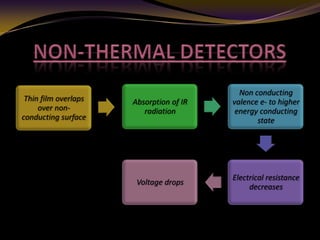
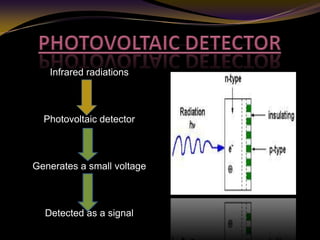
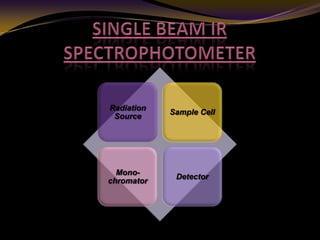
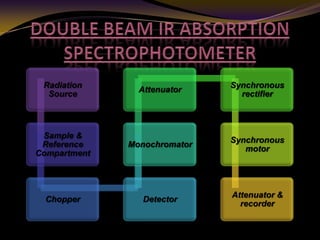
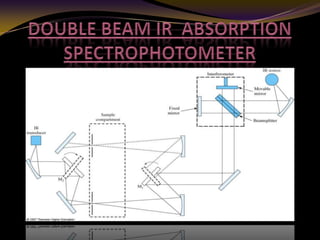
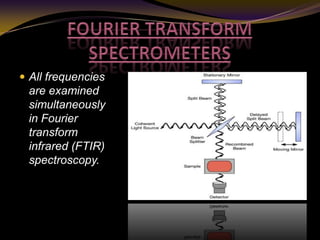
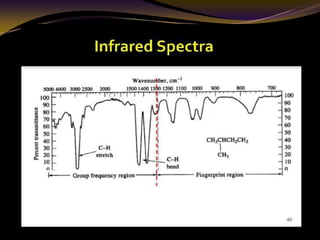
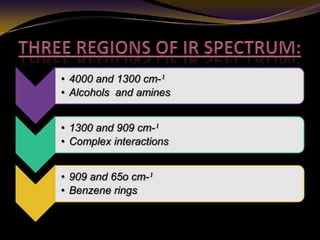
The document discusses infrared (IR) absorption spectroscopy. It begins by defining IR spectroscopy and explaining that it deals with the infrared region of the electromagnetic spectrum. It then discusses the different IR regions and how IR radiation causes molecular vibrations when it hits a molecule. The document goes on to describe different types of molecular vibrations including stretching, bending, scissoring, and twisting vibrations. It also discusses factors that affect vibrational frequencies such as atomic mass and bond strength. Finally, it briefly discusses instrumentation used in IR spectroscopy such as sources, sample cells, detectors, and the applications of IR spectroscopy.