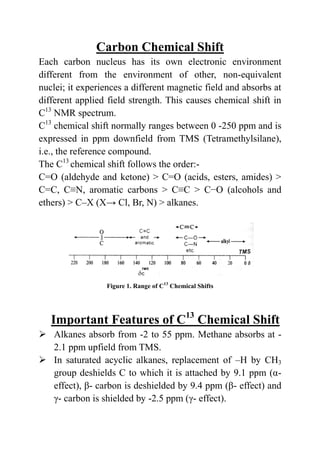
C13 NMR spectroscopy provides information about carbon structures. It detects the less abundant C13 isotope. Though less sensitive than proton NMR, C13 NMR spectra are easier to interpret due to fewer splitting patterns. Each non-equivalent carbon absorbs at a different chemical shift depending on its electronic environment. Chemical shifts typically range from 0-250 ppm downfield from TMS. Factors like hybridization, electronegativity of substituents, and substituent effects influence the chemical shift. C13 NMR is useful for determining carbon skeletons and functional groups.