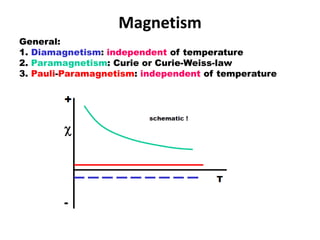
1. The document discusses magnetism in transition metals and lanthanides. It explains different types of magnetism including diamagnetism, paramagnetism, and Pauli paramagnetism.
2. For transition metals, magnetism arises mainly from partially filled d-orbitals. The magnetic moment can provide information about the number of unpaired electrons, high-spin vs low-spin complexes, spectral behavior, and structure.
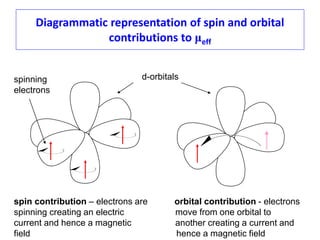
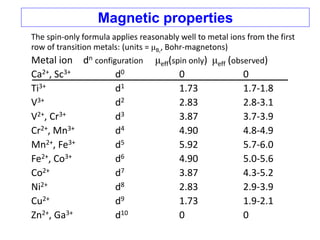
3. Both spin and orbital contributions give rise to magnetic moments. The spin-only formula is discussed and applied to examples. Orbital contributions are also explained.

![Sources of Paramagnetism
Orbital motion of the electron generates ORBITAL MAG. MOMENT (μl)
Spin motion of the electron generates SPIN MAG. MOMENT (μs)
l = orbital angular momentum; s = spin angular momentum
For multi-electron systems
L = l1 + l2 + l3 + …………….
S = s1 + s2 + s3 + ……………
μl+s = [4S(S+1) + L(L+1)]1/2 Β.Μ.
For TM-complexes, the magnetic properties arise mainly from the exposed d-
orbitals. The d-orbitals are perturbed by ligands.
∴ The rotation of electrons about the nucleus is restricted
which leads to L = 0
μs = [4S(S+1)]1/2 Β.Μ.
S = n (1/2) = n/2; n = no of unpaired electrons
Hence
μs = [4(n/2)(n/2+1)]1/2Β.Μ.
= [n(n+2)]1/2 Β.Μ.
This is called Spin-Only Formula
μs = 1.73, 2.83, 3.88, 4.90, 5.92 BM for n = 1 to 5, respectively](https://image.slidesharecdn.com/2-220526132859-885dadbe/85/2-Magnetochemistry-M-Sc-I-Part-II-pptx-4-320.jpg)
![Example:
What is the magnetic susceptibility of [CoF6]3-, assuming
that the spin-only formula will apply:
[CoF6]3- is high spin Co(III). (you should know this). High-
spin Co(III) is d6 with four unpaired electrons, so n = 4.
We have μeff = n(n + 2)
= 4.90 μB
eg
t2g
energy
high spin d6 Co(III)](https://image.slidesharecdn.com/2-220526132859-885dadbe/85/2-Magnetochemistry-M-Sc-I-Part-II-pptx-7-320.jpg)

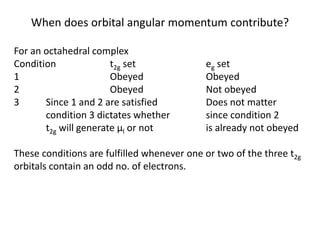
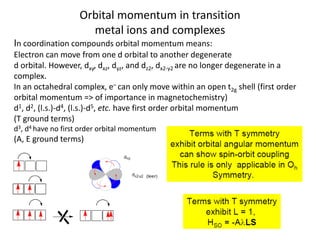
![Example: Given that the value of the spin-orbit coupling
constant λ, is -316 cm-1 for Ni2+, and Δoct is 8500 cm-1,
calculate μeff for [Ni(H2O)6]2+. (Note: for an A ground state α =
4, and for an E ground state α = 2).
High-spin Ni2+ = d8 = A ground state, so α = 4.
n = 2, so μ(spin only) = (2(2+2))0.5 = 2.83 μB
μeff = μ(spin only)(1 - (-316 cm-1 x (4/8500 cm-1)))
= 2.83 μB x 1.149
= 3.25 μB
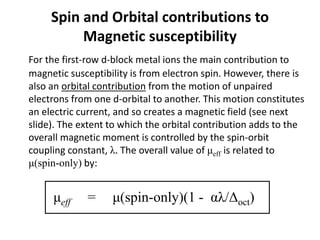
Spin and Orbital contributions to
Magnetic susceptibility](https://image.slidesharecdn.com/2-220526132859-885dadbe/85/2-Magnetochemistry-M-Sc-I-Part-II-pptx-12-320.jpg)
![The value of λ is negligible for very light atoms, but increases
with increasing atomic weight, so that for heavier d-block
elements, and for f-block elements, the orbital contribution is
considerable. For 2nd and 3rd row d-block elements, λ is an
order of magnitude larger than for the first-row analogues.
Most 2nd and 3rd row d-block elements are low-spin and
therefore are diamagnetic or have only one or two unpaired
electrons, but even so, the value of μeff is much lower than
expected from the spin-only formula. (Note: the only high-spin
complex from the 2nd and 3rd row d-block elements is [PdF6]4-
and PdF2).
Spin and Orbital contributions to
Magnetic susceptibility](https://image.slidesharecdn.com/2-220526132859-885dadbe/85/2-Magnetochemistry-M-Sc-I-Part-II-pptx-13-320.jpg)
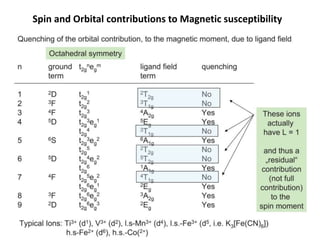
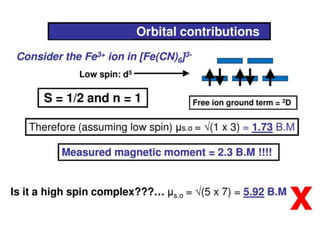
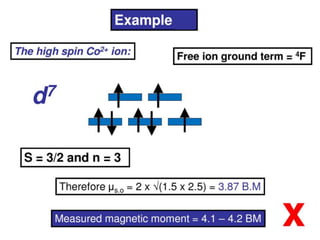
![Example
Oh
Td
Free ion
NiII (d8)
S = 1, L = 3
L+S = [4S(S+1)+L(L+1)]1/2 = 4.47 B.M.
Orbital Contribution = 0
The magnetic moment
is close to spin only
value
Magnetic moment is
higher than the spin-only
value as there is positive
orbital contribution](https://image.slidesharecdn.com/2-220526132859-885dadbe/85/2-Magnetochemistry-M-Sc-I-Part-II-pptx-17-320.jpg)
