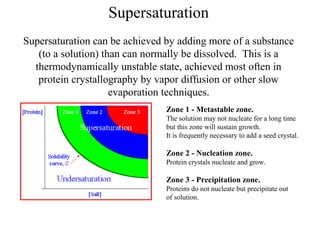
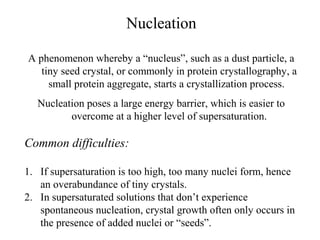
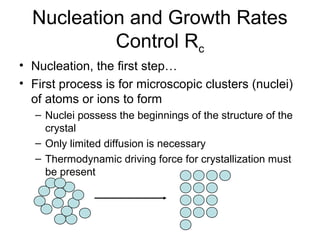
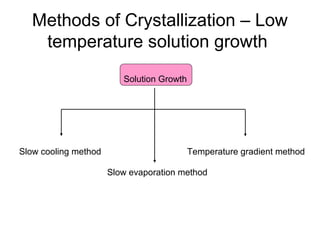
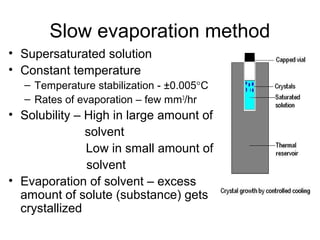
This document discusses various methods for crystal growth, including growing crystals from solution and vapor phase. It describes how crystallization occurs as atoms or molecules arrange in a repeating pattern. There are multiple techniques for obtaining crystals depending on the material, such as growing from molten solid, solution, or vapor phase. A common method is growing from solution, which involves precipitating crystals from a saturated solution by techniques like cooling or evaporation to reduce solubility in a controlled manner. Proper conditions like solvent choice, temperature control, and supersaturation levels are important for successful crystal growth.