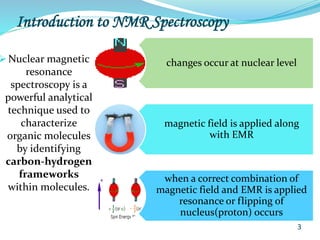
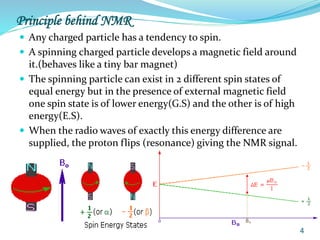
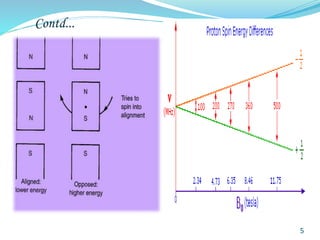
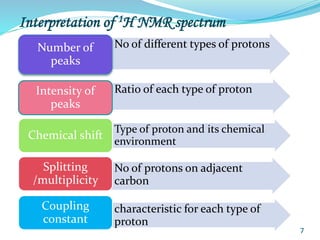
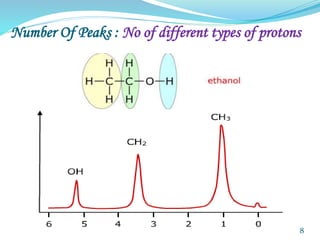
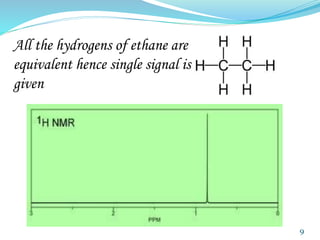
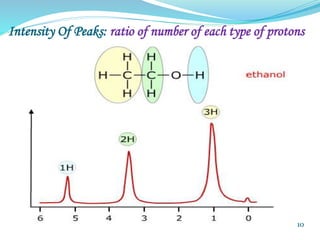
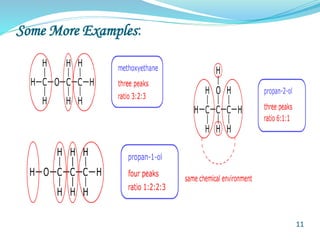
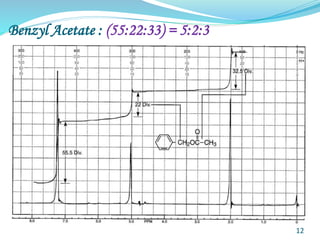
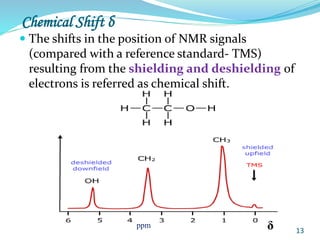
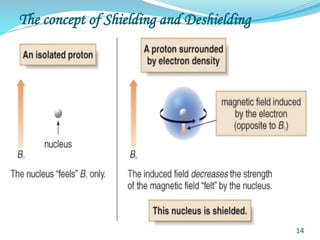
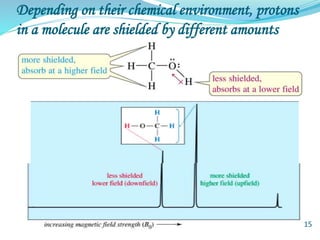
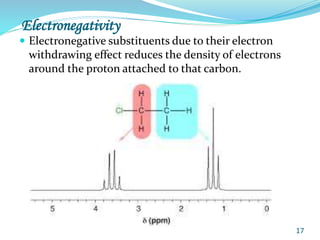
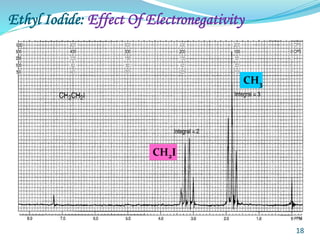
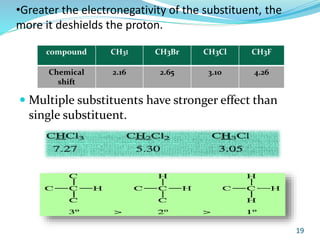
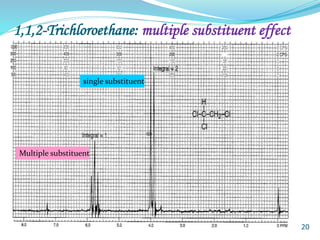
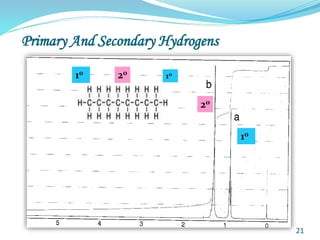
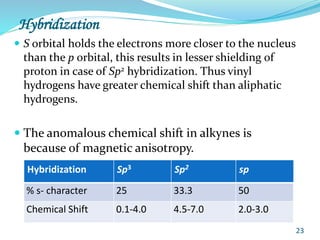
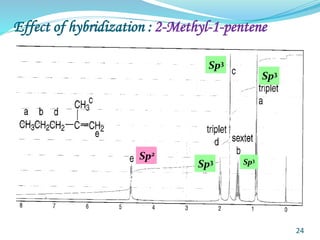
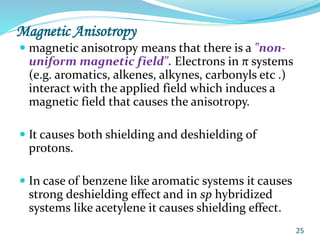
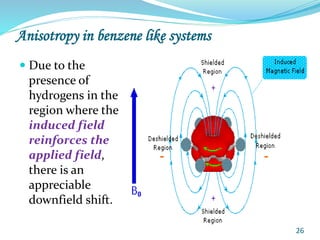
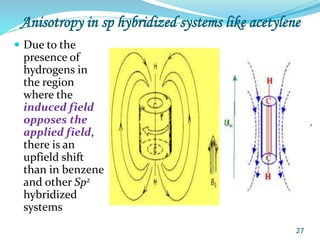
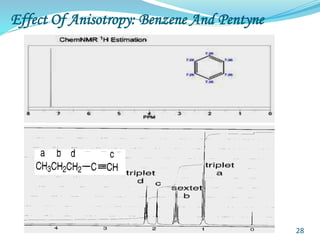
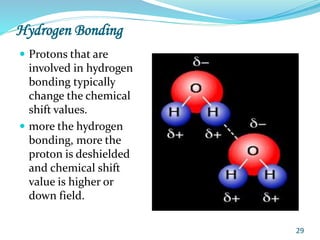
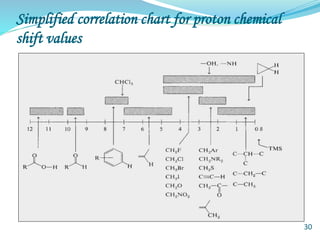
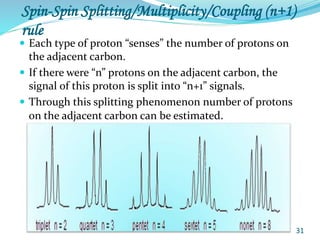
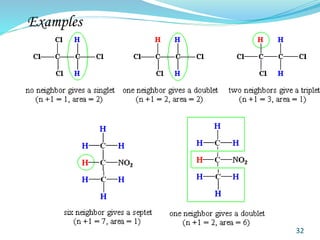
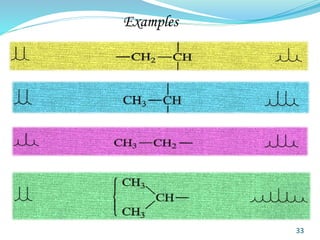
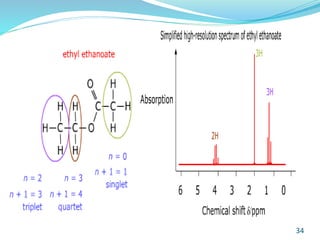
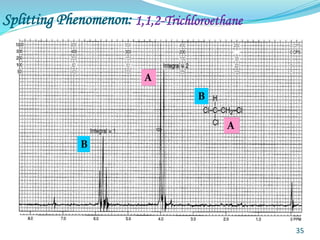
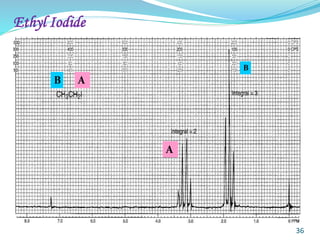
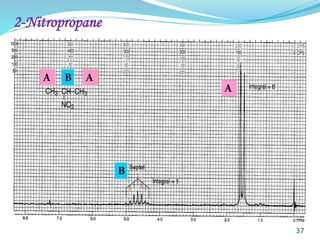
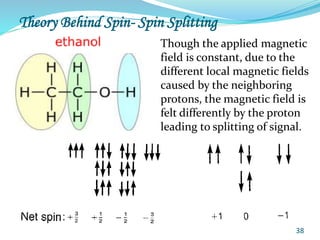
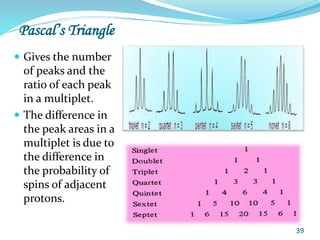
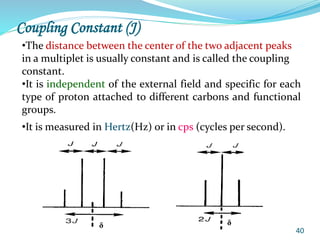
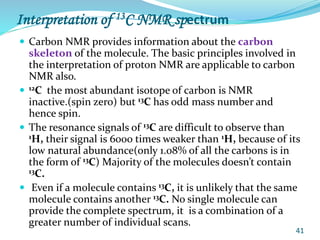
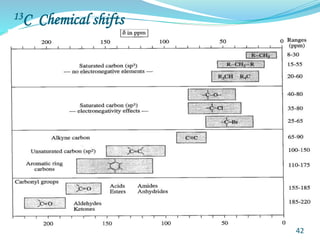
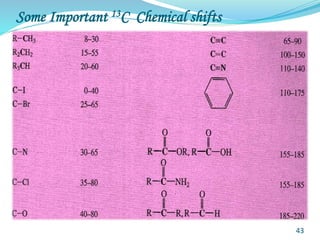
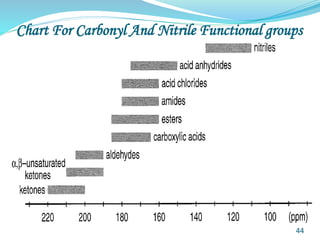
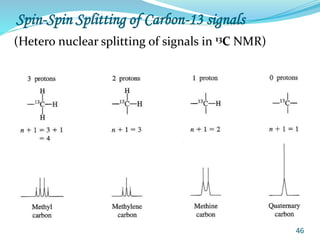
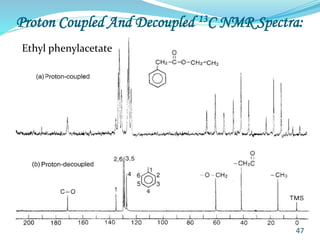
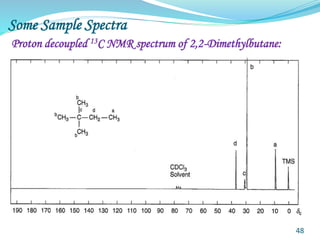
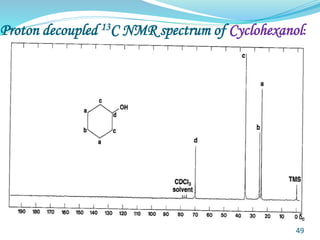
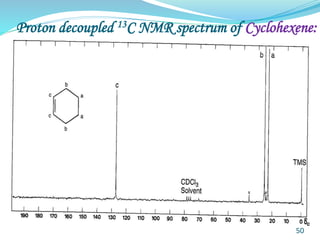
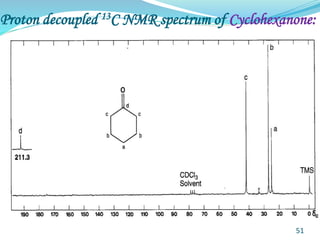
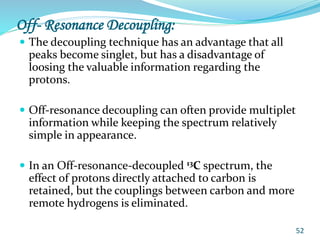
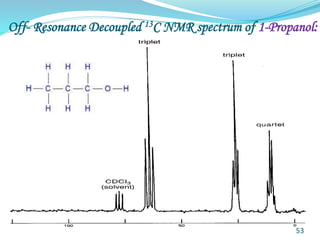
This document provides an introduction to NMR spectroscopy and its principles. It discusses the two main types of NMR - proton (1H NMR) and carbon-13 (13C NMR) spectroscopy. It covers the interpretation of 1H NMR spectra, including number of peaks, intensity of peaks, chemical shift, spin-spin splitting/multiplicity, and coupling constants. Interpretation of 13C NMR spectra is also discussed, including chemical shifts and spin-spin splitting. Examples of spectra are provided to illustrate these concepts. The document concludes that NMR spectroscopy is an effective tool for determining molecular structure.