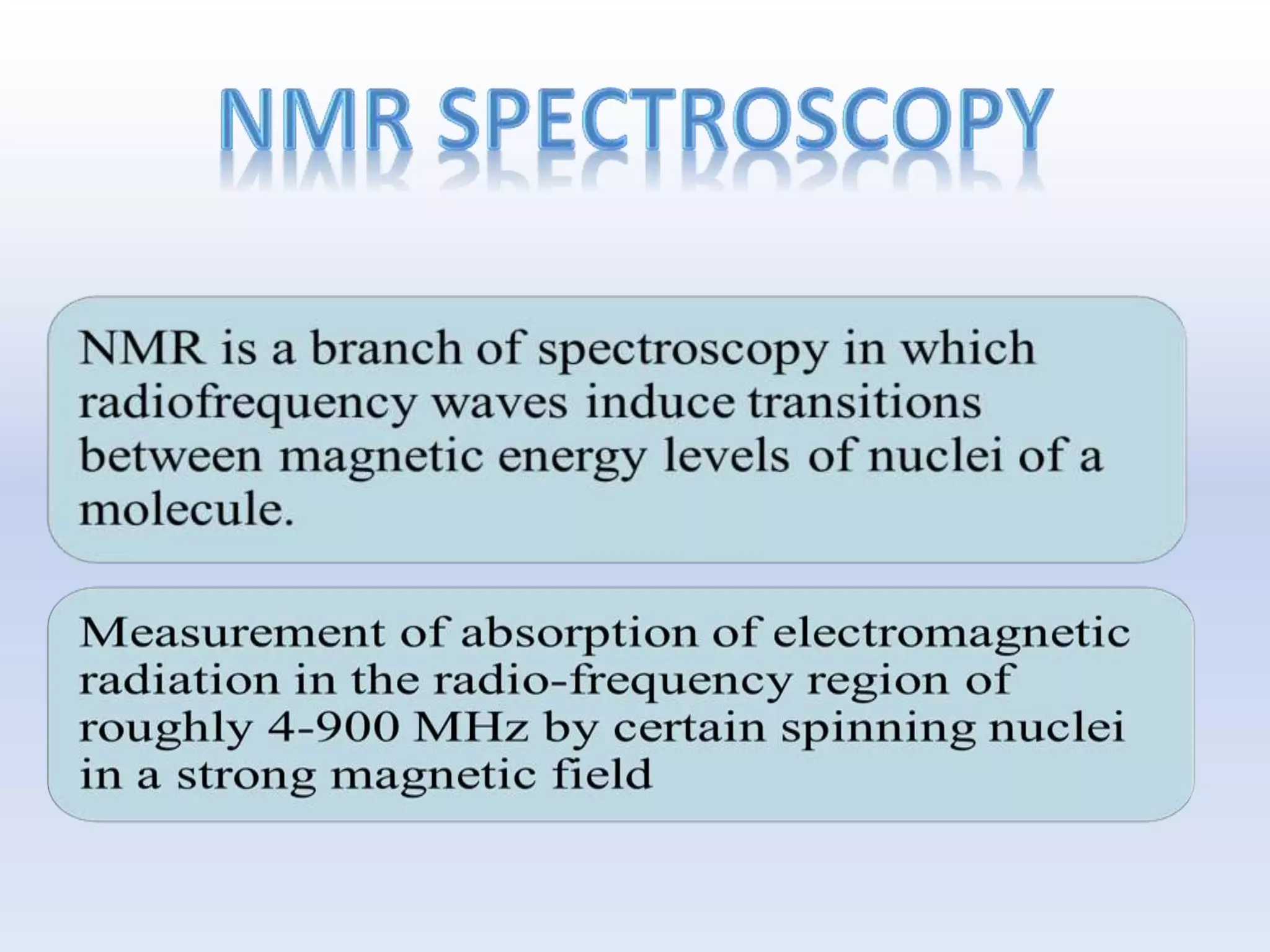
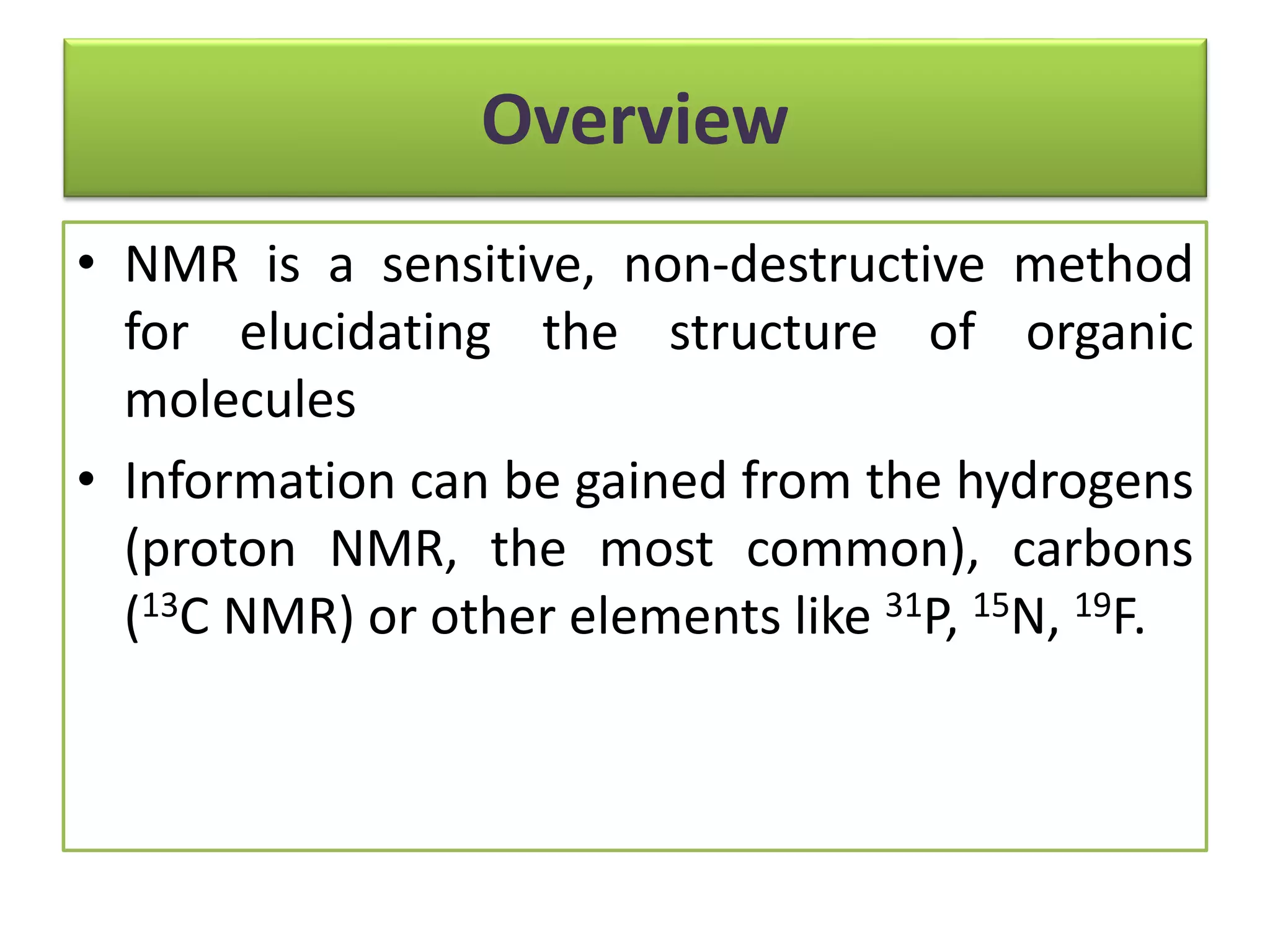
NMR is a sensitive, non-destructive method for elucidating the structure of organic molecules. Information can be gained from protons, carbons, and other elements. There are two main types of NMR: 1D NMR and 2D NMR, which plots data in a space defined by two frequency axes rather than one. Common types of 2D NMR include COSY, NOESY, and EXSY. NMR signals provide information about the number, environment, and connectivity of different nuclei in a molecule.




















![NMR Spectrum- Interpretation
Number of signals: Indicates how many different kinds of
protons are present
Position of signals: Indicates Magnetic environment of the
signal
Relative Intensity: Proportional to number of protons present
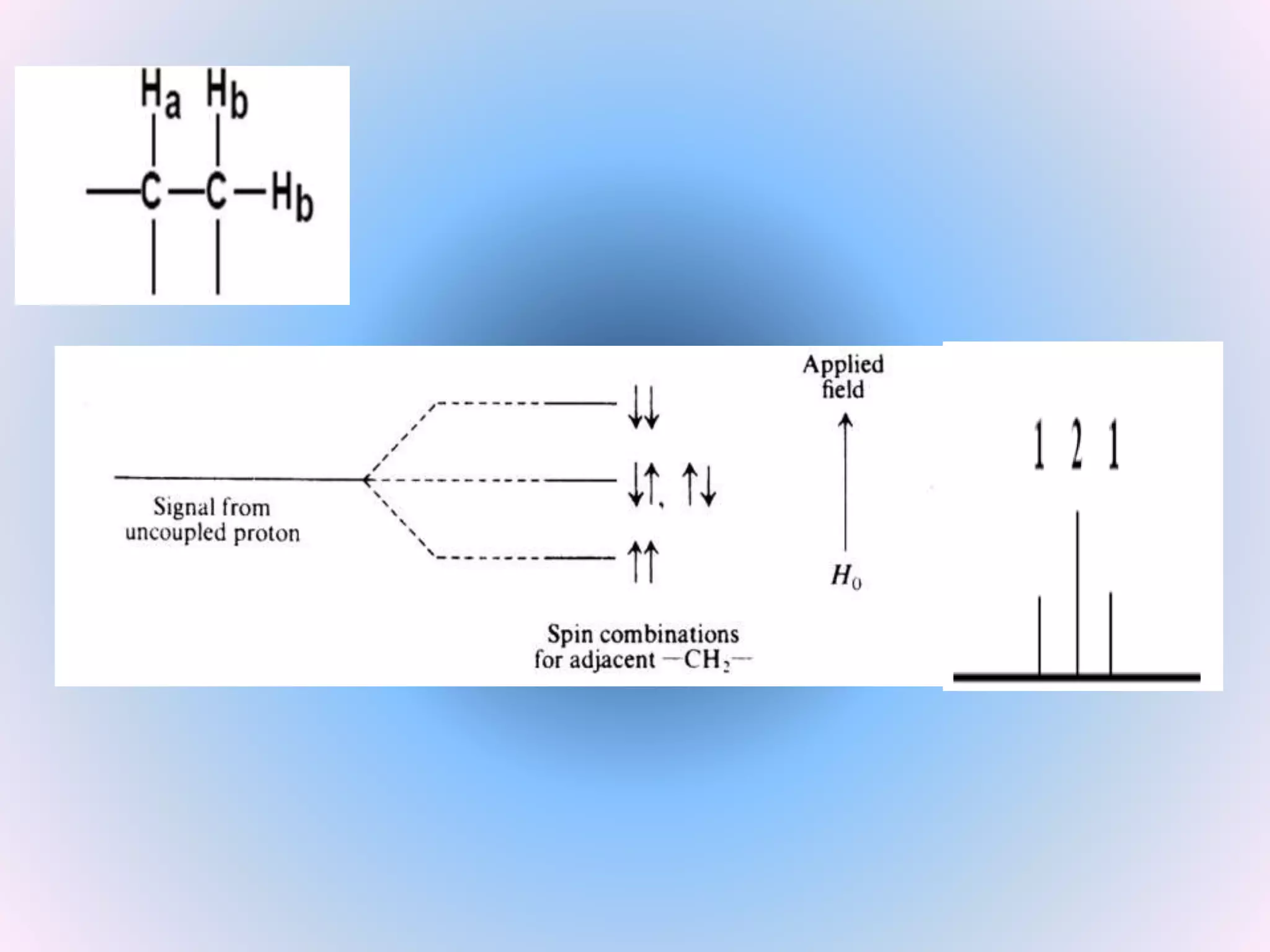
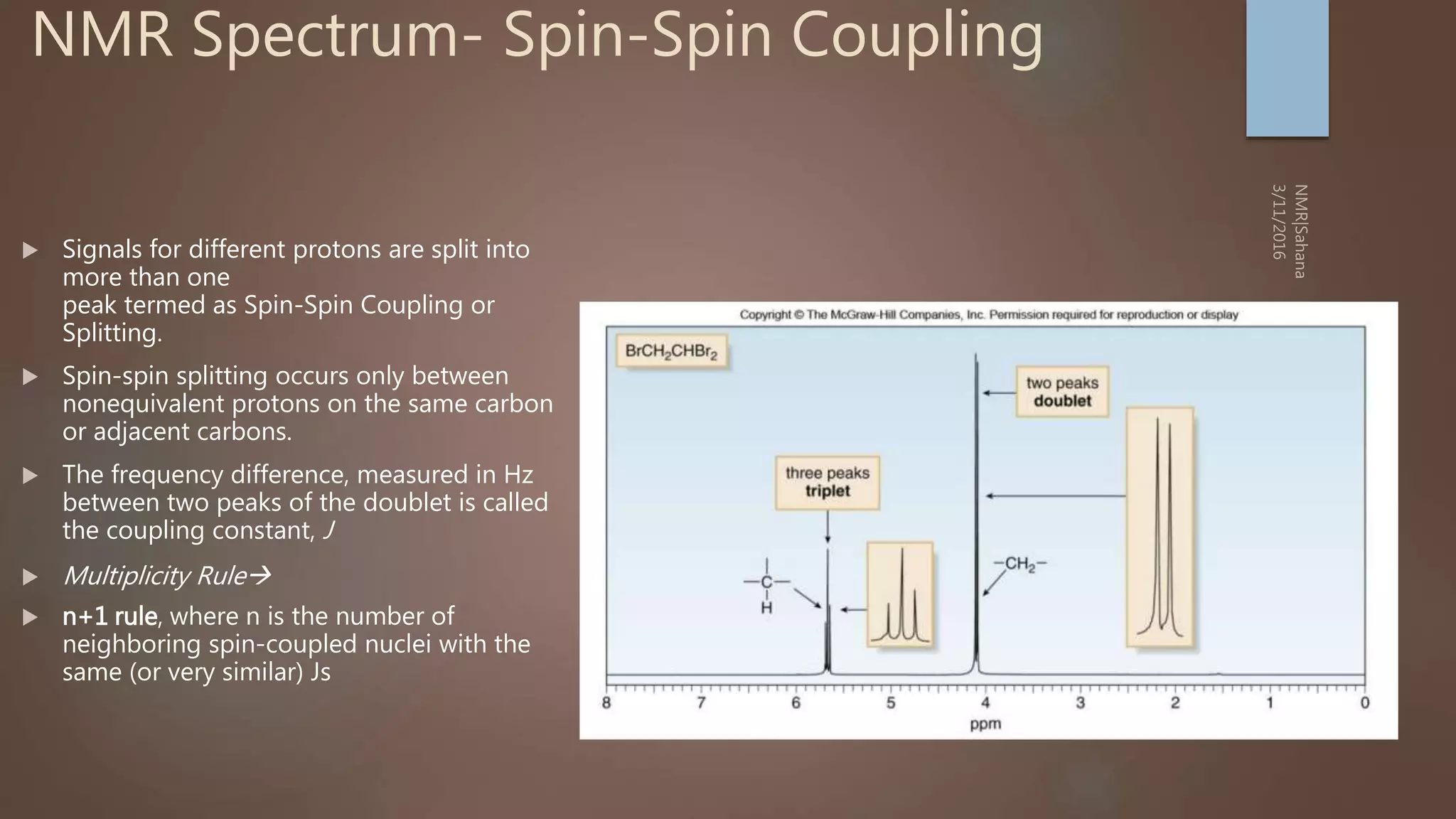
Splitting: Indicates number of splitting Nuclei [Usually
Protons]](https://image.slidesharecdn.com/nmr-basic-1d-190625082042/75/Basic-NMR-25-2048.jpg)































![The frequency ν at which energy is absorbed or emitted is given by Bohr̕ s
relationship:
ν = E2 – E1 / h
ν = -1/2 [ γ h / 2π ] B0 + 1/2 [ γ h / 2π ] B0 / h
ν = (γ / 2π )B0
This is the Larmor equation which is the mathematical basis for NMR.](https://image.slidesharecdn.com/nmr-basic-1d-190625082042/75/Basic-NMR-57-2048.jpg)




