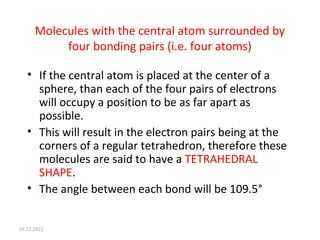
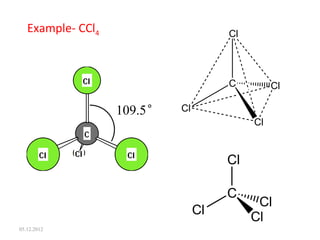
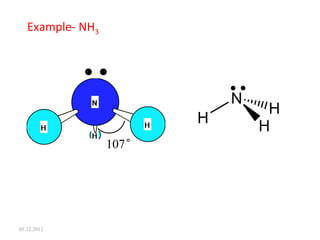
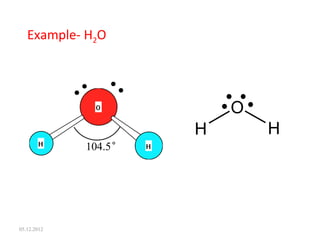
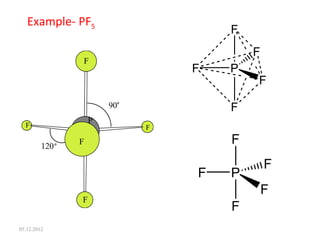
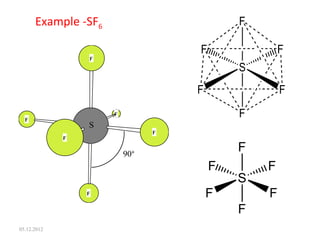
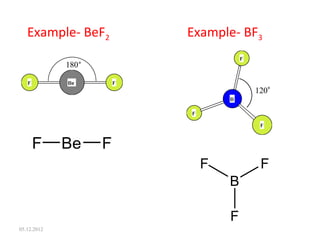
The VSEPR (Valence Shell Electron Pair Repulsion) theory predicts molecular geometry based on electron pair repulsion. The shape is determined by drawing a Lewis diagram and counting bonding and non-bonding electron pairs. Common molecular shapes include tetrahedral for 4 electron pairs, trigonal pyramidal for 3 bonds and 1 lone pair, bent for 2 bonds and 2 lone pairs, and trigonal planar for 3 bonds. Angles vary based on number of lone pairs. Examples of molecular shapes are given for molecules like CH4, NH3, H2O, PF5 and SF6.