1. The document provides information on molecular structure and bonding theories including atomic and molecular orbitals, linear combination of atomic orbitals, molecular orbital diagrams of diatomic molecules like N2, O2 and F2, π molecular orbitals of butadiene and benzene, and crystal field theory.
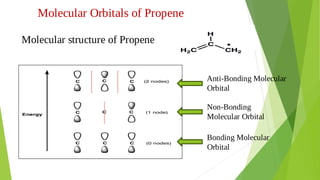
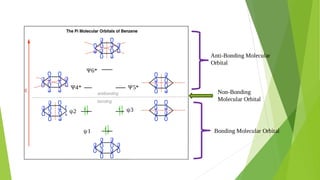
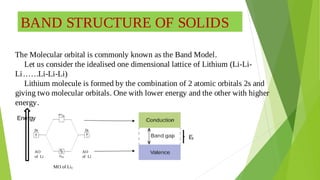
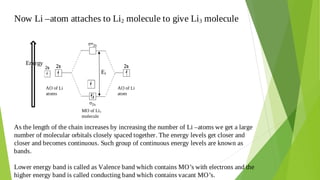
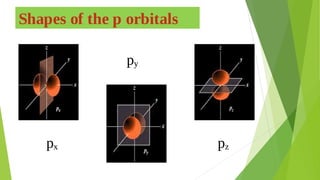
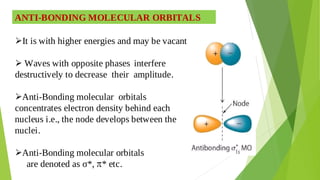
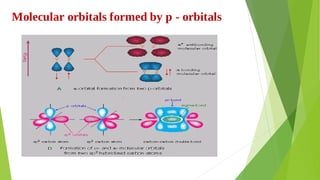
2. Key concepts covered include how molecular orbitals are formed from the overlap and combination of atomic orbitals, bonding and anti-bonding molecular orbitals, and how molecular orbital diagrams can be used to explain bonding properties.
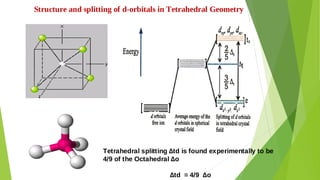
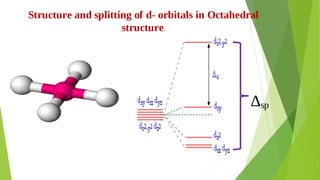
3. Crystal field theory is introduced as explaining the color, magnetic properties and other characteristics of crystalline substances based on interactions between the d-orbitals of metal ions and ligand ions or molecules




















![Relation between Electronic Configuration and Molecular behaviour
1. Stability of Molecules:
Let Nb = No. of electrons present in the bonding orbitals
Na = No. of electrons present in the anti-bonding orbitals
If Nb > Na then molecule is stable because of the more net force of attraction.
If Nb < Na then molecule is unstable because of the more net repulsive forces.
If Nb = Na then also molecule is unstable because the influence of electrons in
anti-bonding orbitals is more than the electrons in the bonding orbitals.
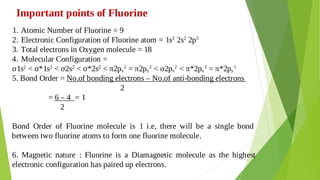
2. Bond Order:
It is defined as the half of the difference between the number of the electrons
present in bonding and anti-bonding orbitals.
Bond order = [ Nb – Na]
2
If Nb > Na then bond order is positive and the molecule is said to be stable.
If Nb < Na then bond order is negative and the molecule is said to be unstable.
Stability is directly proportional to the bond order.
Bond length decreases with increase in bond order
Bond energy is directly proportional to bond order.](https://image.slidesharecdn.com/unit1-230818061846-8c9c5762/85/Unit-1-pdf-24-320.jpg)