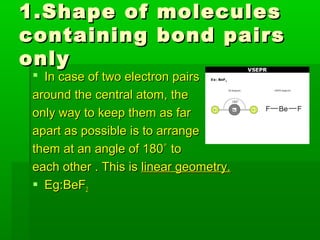
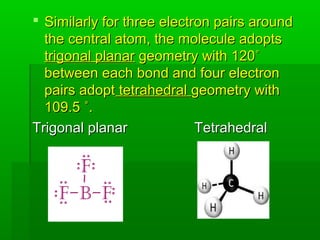
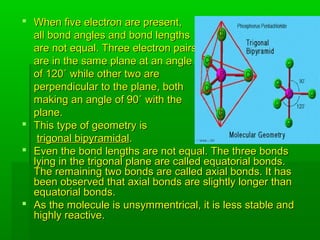
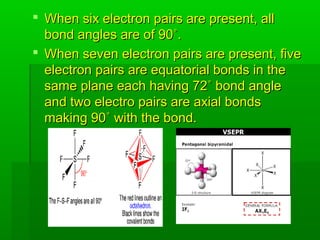
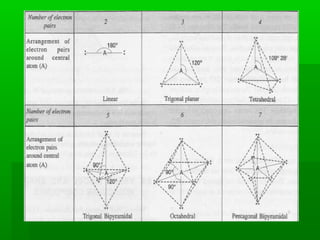
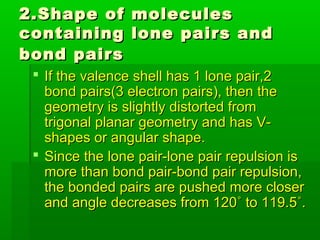
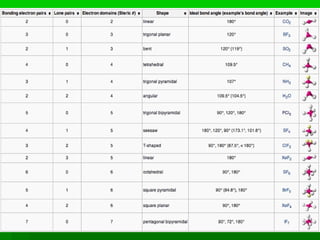
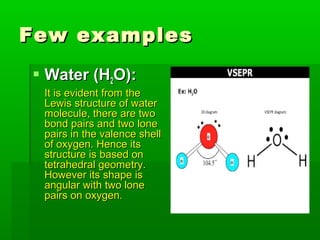
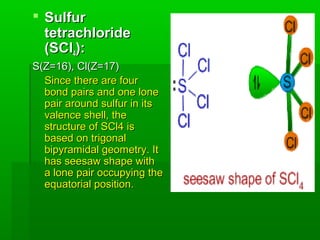
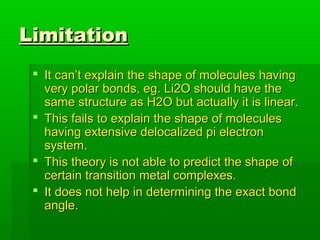
VSEPR theory posits that the geometry of a molecule is determined by the arrangement of electron pairs around a central atom, which minimizes repulsion between them. The theory categorizes molecular shapes based on the number of bond and lone pairs, leading to distinct geometries such as linear, trigonal planar, tetrahedral, and others. However, the model has limitations, including its inability to explain the shapes of certain polar molecules and transition metal complexes.