Ligand Field Theory was postulated in the 1950s as a modification of crystal field theory and molecular orbital theory. It can explain the geometry of coordination compounds like octahedral, tetrahedral, and square planar using crystal field theory. However, ligand field theory also considers sigma and pi bonding, which are important for understanding the behavior of neutral ligands like carbon monoxide and the strong field ligands carbon monoxide and cyanide.


![Ligand Field Theory
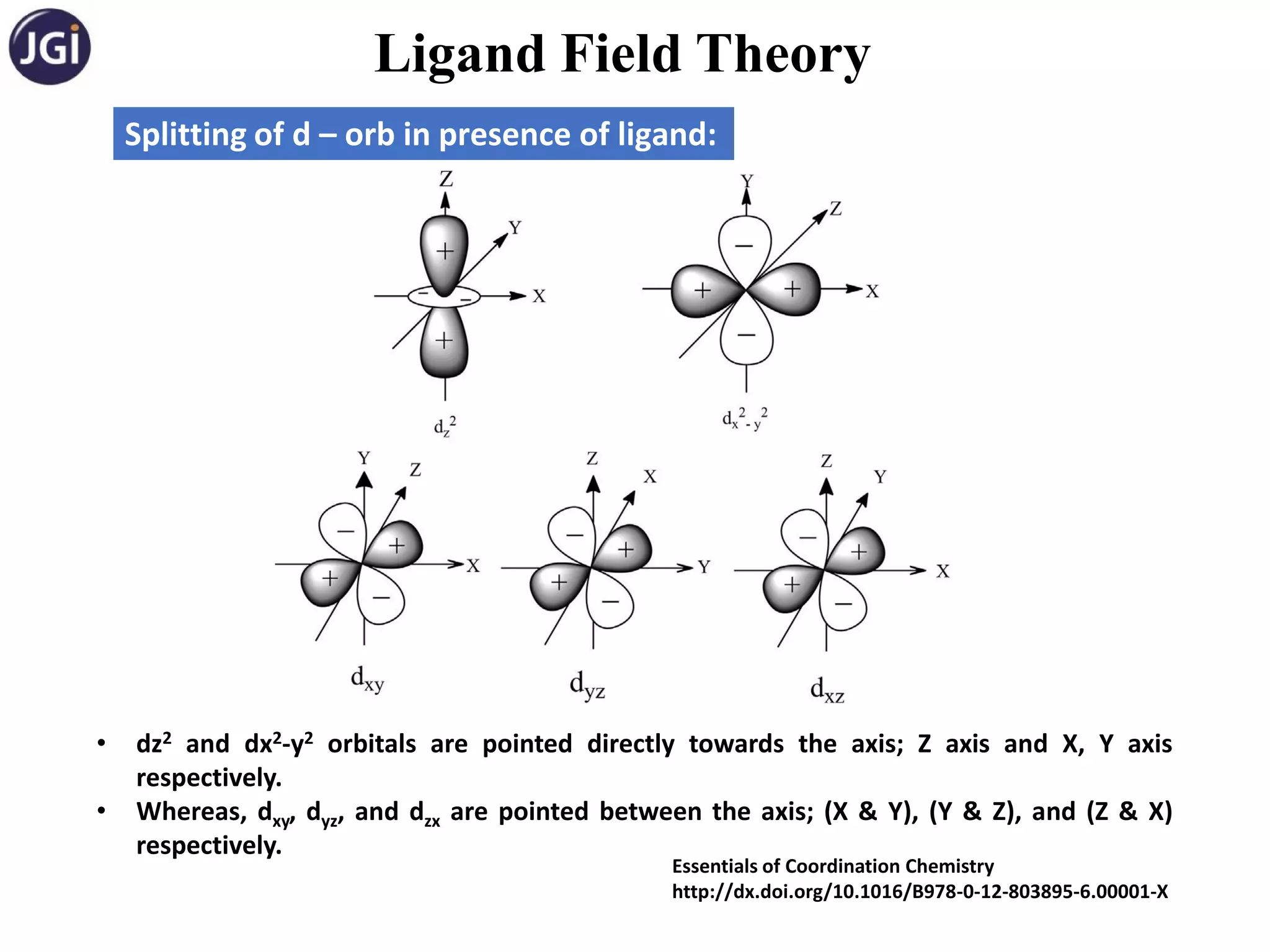
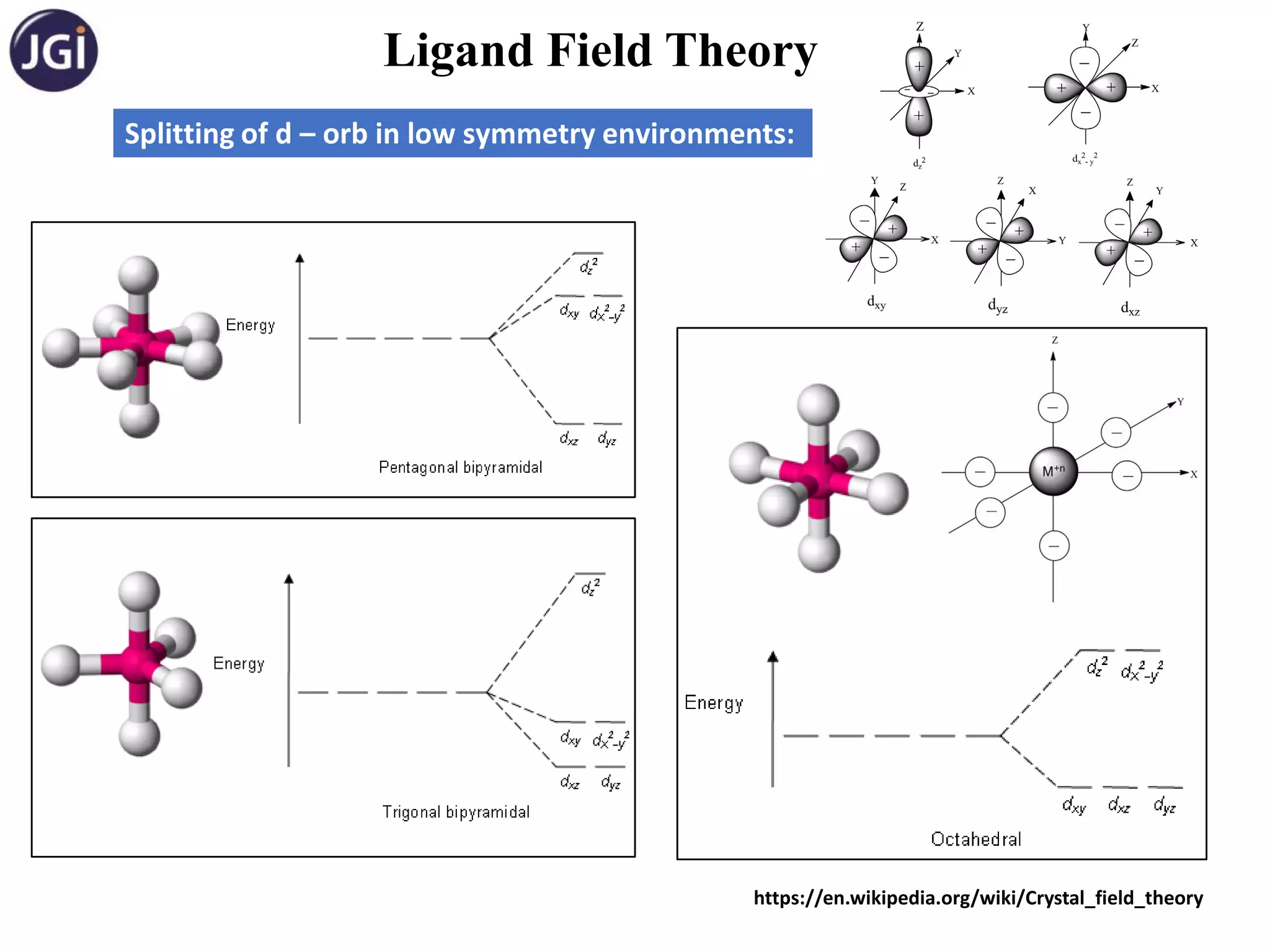
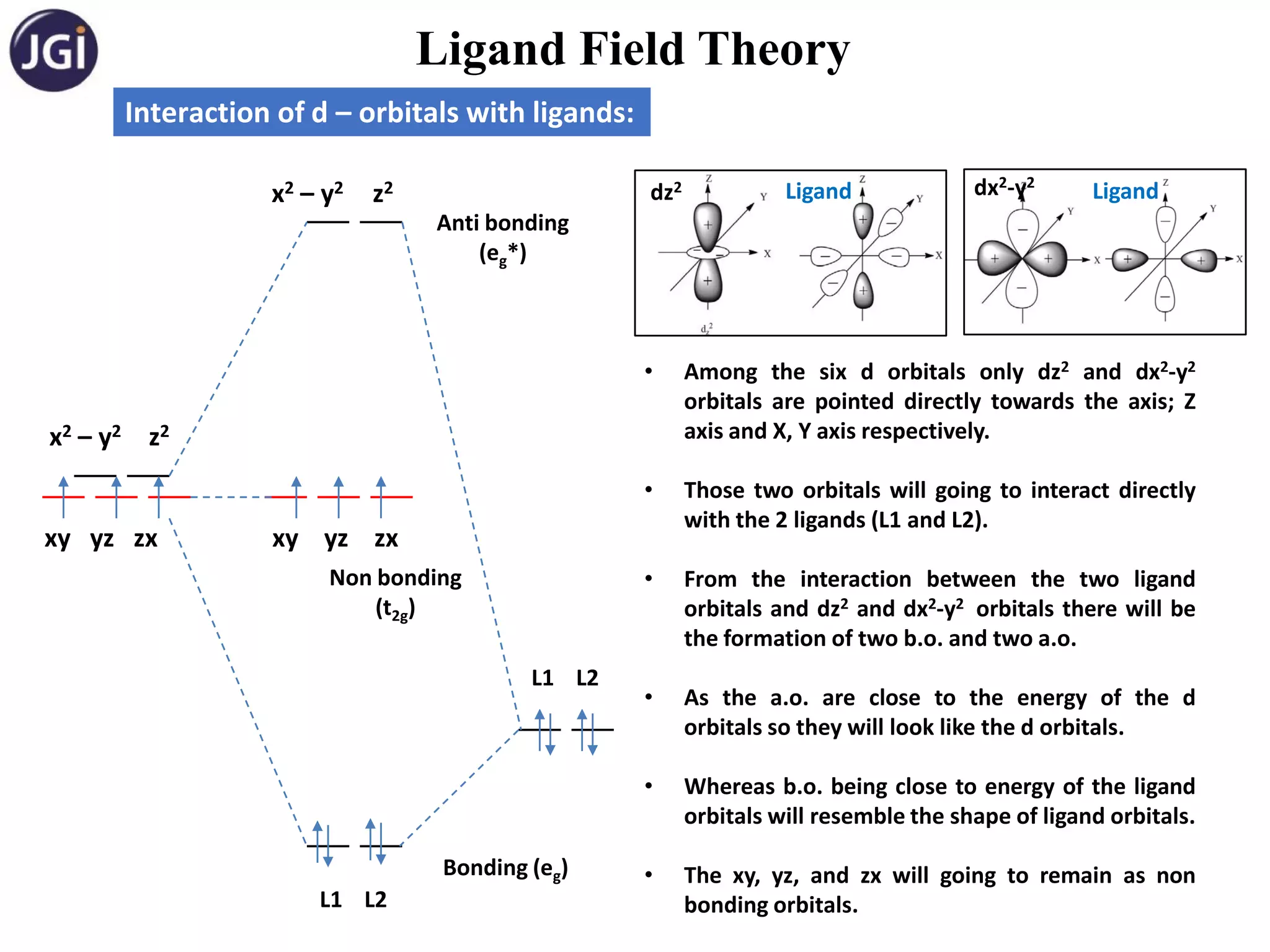
Splitting of d – orb in octahedral complexes:
Approach of ligand in octahedral field:
Pointed directly towards
the approach of 6 ligands
Not in the same
directions as ligands
5 degenerate
d – orbitals of
free ions
Splitting of d
– orbitals in
octahedral
field
• dz2 and dx2-y2 orbitals (eg) are
higher in energy; Energy gap =
3/5 ΔO
• Whereas, dxy dyz and dzx orbitals
(t2g) are lowered in energy;
Energy gap = 2/5 ΔO
• Example: [Cr(III)Cl6]3-
Repulsion between ligand and metal removes
the degeneracy between the 5 d – orbitals.](https://image.slidesharecdn.com/ligandfieldtheory-supratimchakraborty-220217042537/75/Ligand-field-theory-Supratim-Chakraborty-4-2048.jpg)
![Ligand Field Theory
Splitting of d – orb in tetrahedral complexes:
Far from the approach
of the ligands
More towards
the direction
of the ligands
Approach of ligand in tetrahedral field:
5 degenerate
d – orbitals of
free ions Splitting of d –
orbitals in
tetrahedral field
• dxy dyz and dzx orbitals (t2g) are higher in
energy; Energy gap = 2/5 Δt
• Whereas, dz2 and dx2-y2 orbitals (eg) are
lowered in energy; Energy gap = 3/5 Δt
• Example: [Fe(VI)O4]2-
Unlike octahedral as only 4 ligands are
approaching in tetrahedral so the electrostatic
repulsion is comparatively less and Δt = 4/9 Δo
X
Y
Z
Mn+](https://image.slidesharecdn.com/ligandfieldtheory-supratimchakraborty-220217042537/75/Ligand-field-theory-Supratim-Chakraborty-5-2048.jpg)





![Ligand Field Theory
Δ
Δ Δ
t2g
eg* eg*
eg*
π
π
π*
π*
π orbital
of I -
π* orbital
of CN-
π bonding of ligands with the metal orbitals:
Sigma bonding of ligands
with the metal orbitals
[M(II)(H2O)6]2+ [M(II)(CN)6]4-
[M(II)(I)6]4-
M C O
Metal Ligand back bonding
π orbitals from I- are lower in
energy than metal t2g orbitals. So
the effective and experimental Δ
value becomes smaller. Δ(I-)
π* orbitals from CN- are higher in
energy than metal t2g orbitals. So the
effective and experimental Δ value
becomes bigger. Δ(CO /CN-)
π acceptor ligands
Ligand Metal back bonding
π donor ligands
Absence of any π orbitals and
metal interaction has kept the
value of Δ in standard region.
<
<
Δ(H2O)](https://image.slidesharecdn.com/ligandfieldtheory-supratimchakraborty-220217042537/75/Ligand-field-theory-Supratim-Chakraborty-13-2048.jpg)
