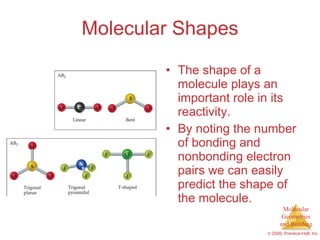
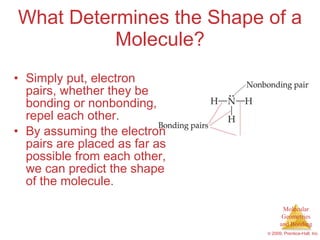
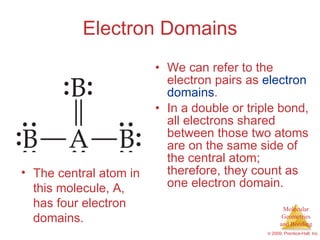
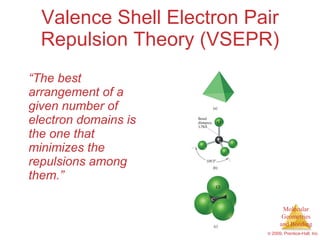
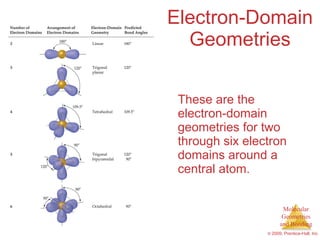
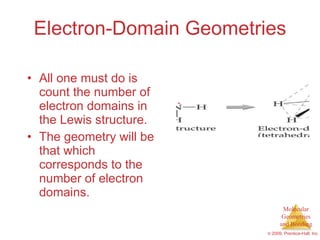
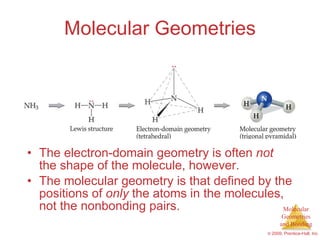
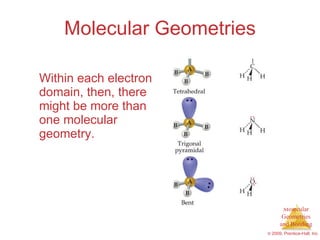
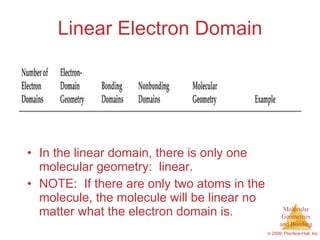
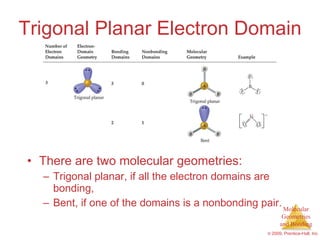
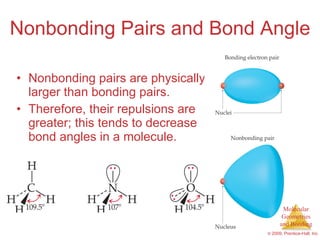
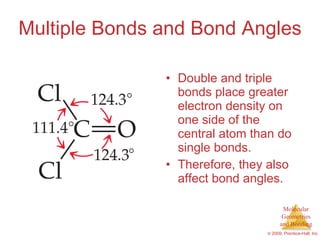
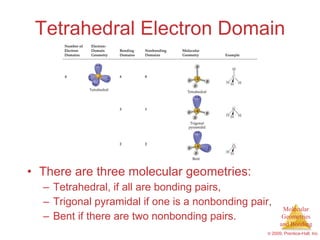
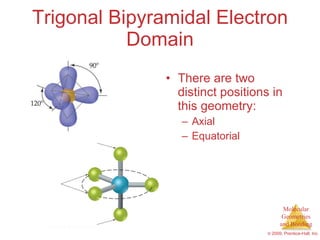
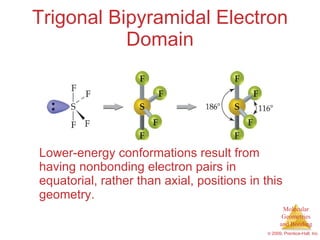
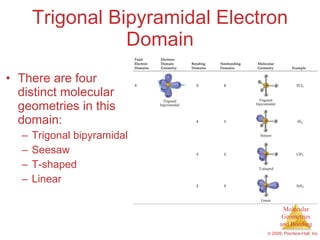
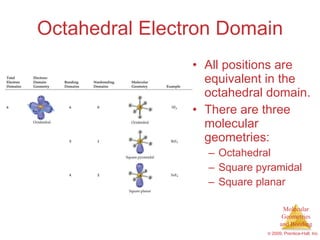
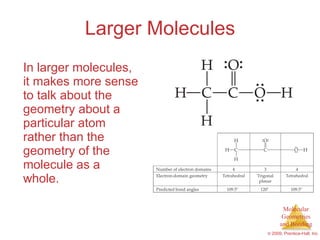
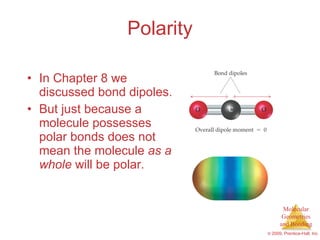
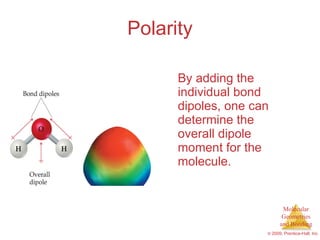
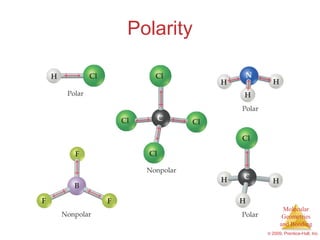
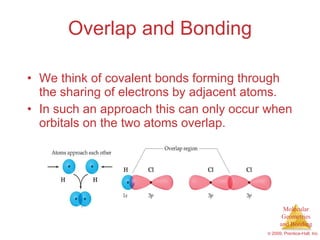
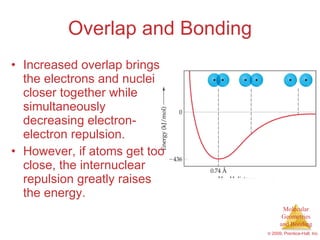
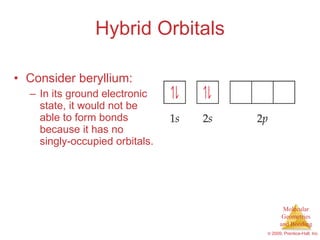
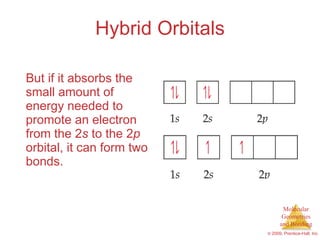
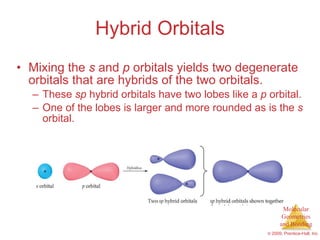
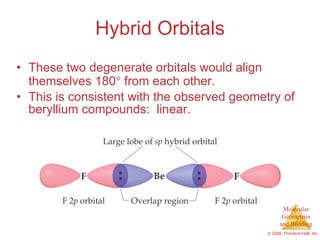
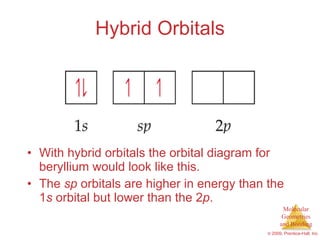
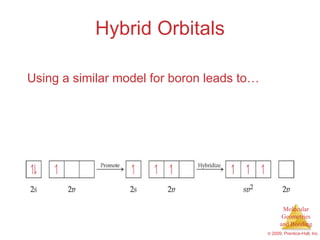
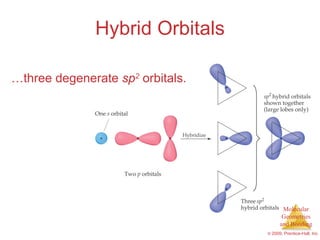
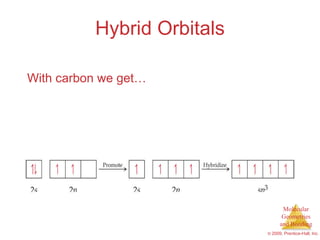
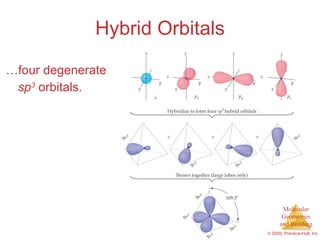
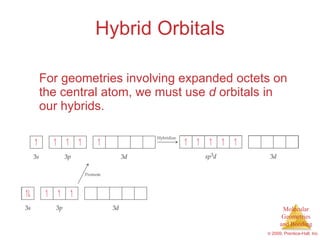
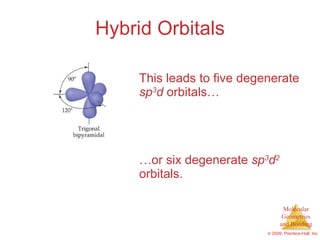
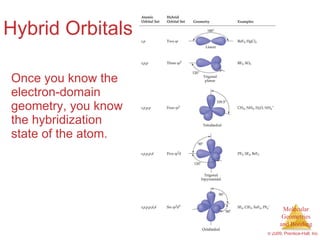
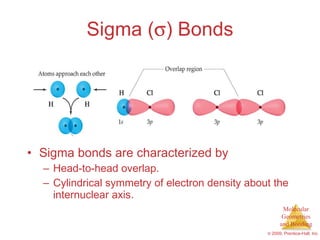
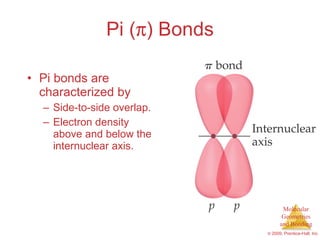
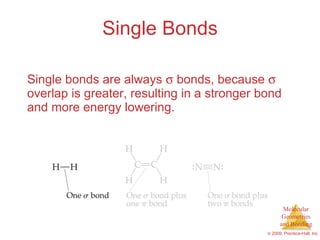
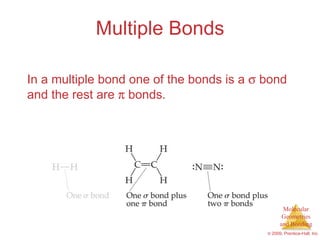
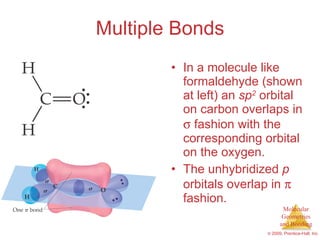
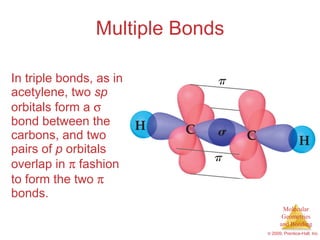
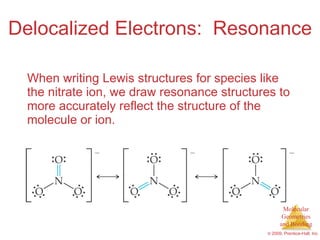
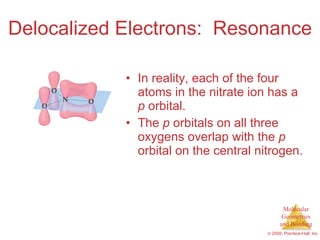
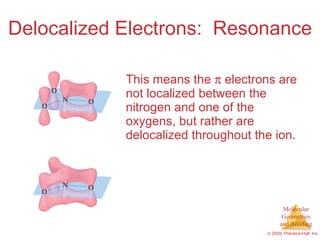
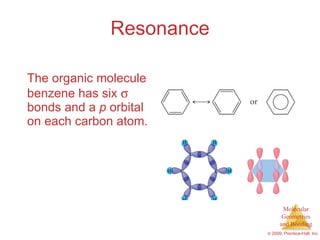
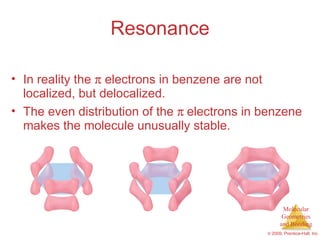
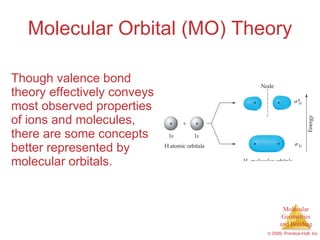
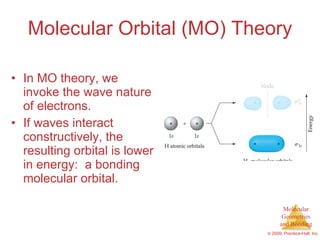
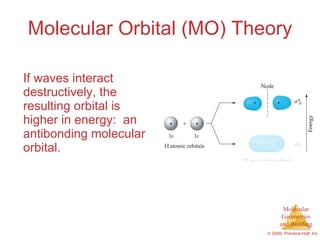
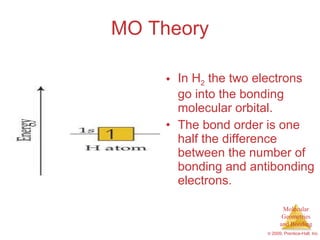
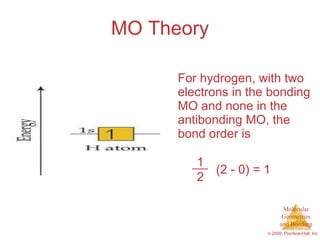
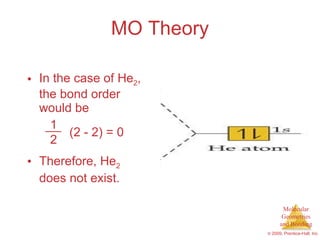
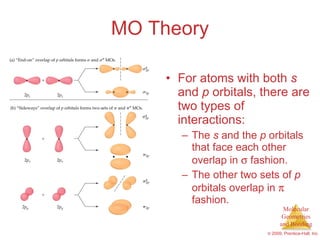
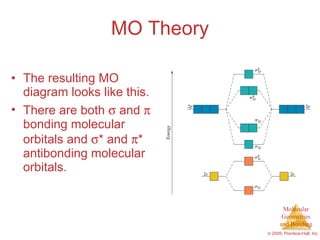
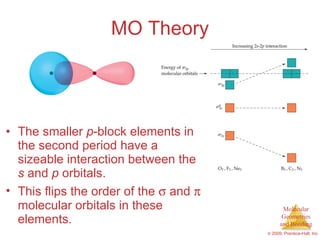
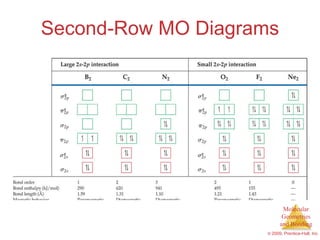
This document provides an overview of molecular shapes and bonding theories. It discusses how the number of electron pairs around an atom determines the electron-domain geometry and molecular geometry. Valence shell electron pair repulsion theory and hybridization are introduced to explain molecular shapes. The document also covers sigma and pi bonding, resonance structures, and molecular orbital theory.