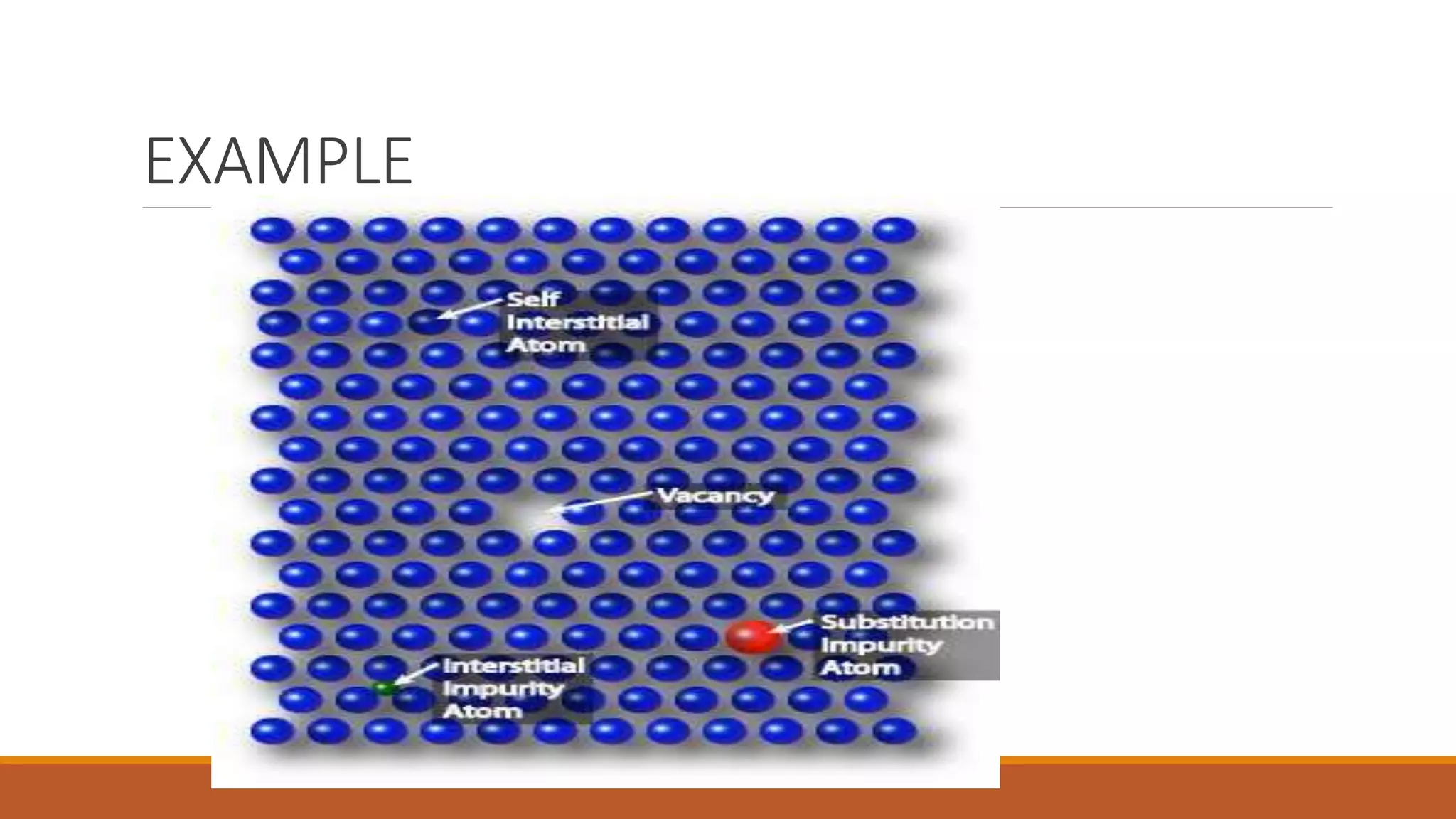
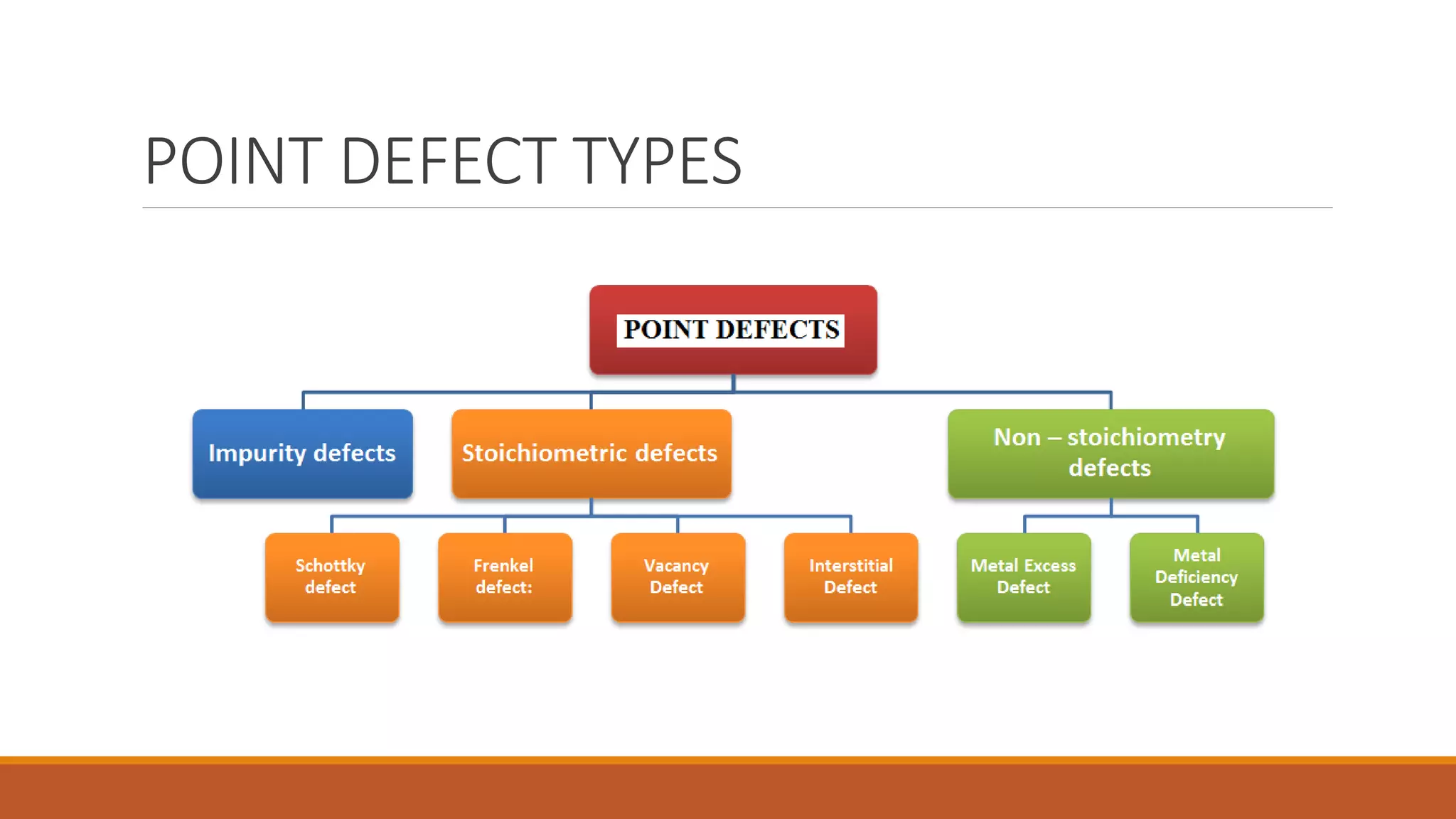
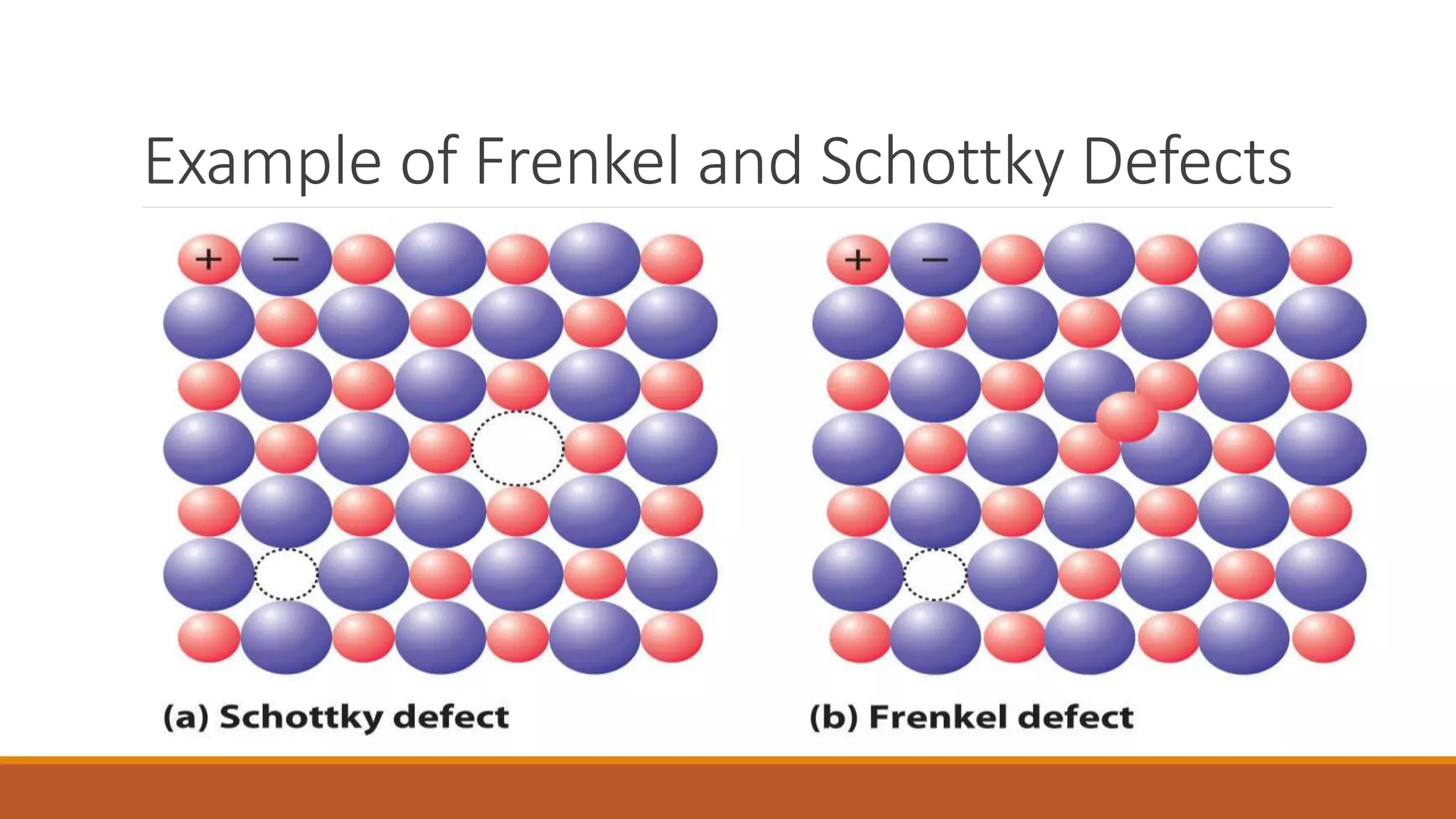
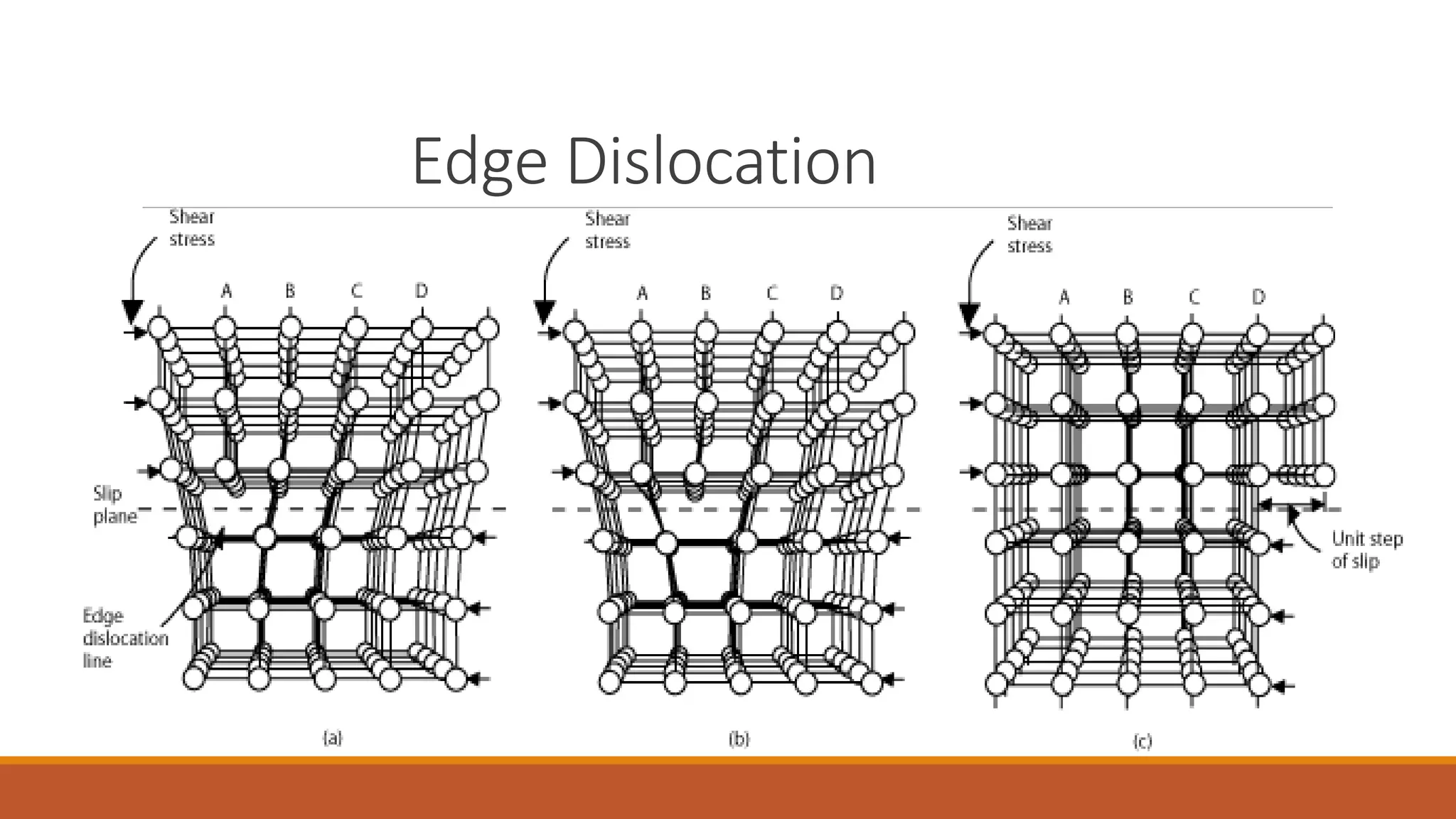
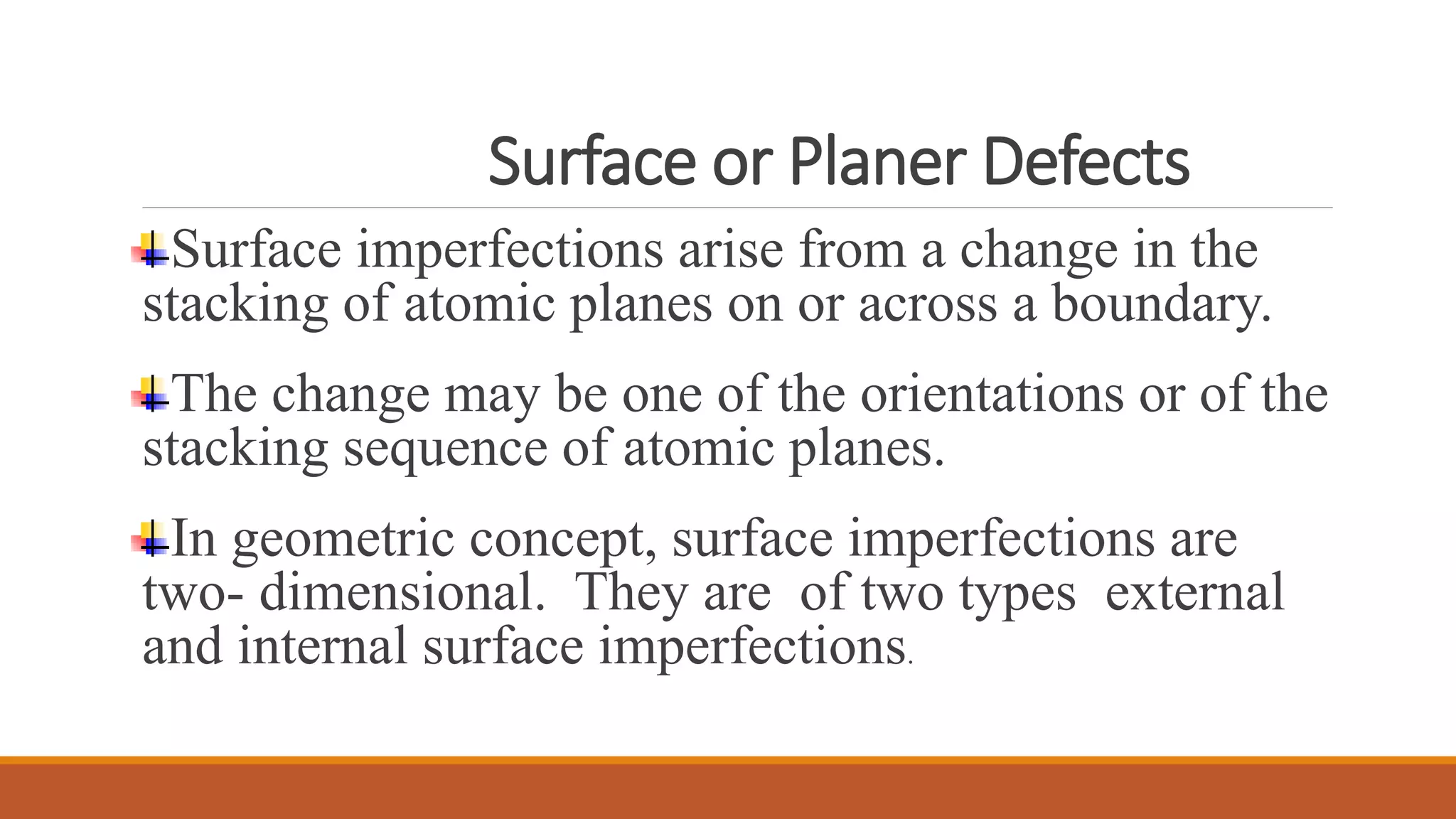
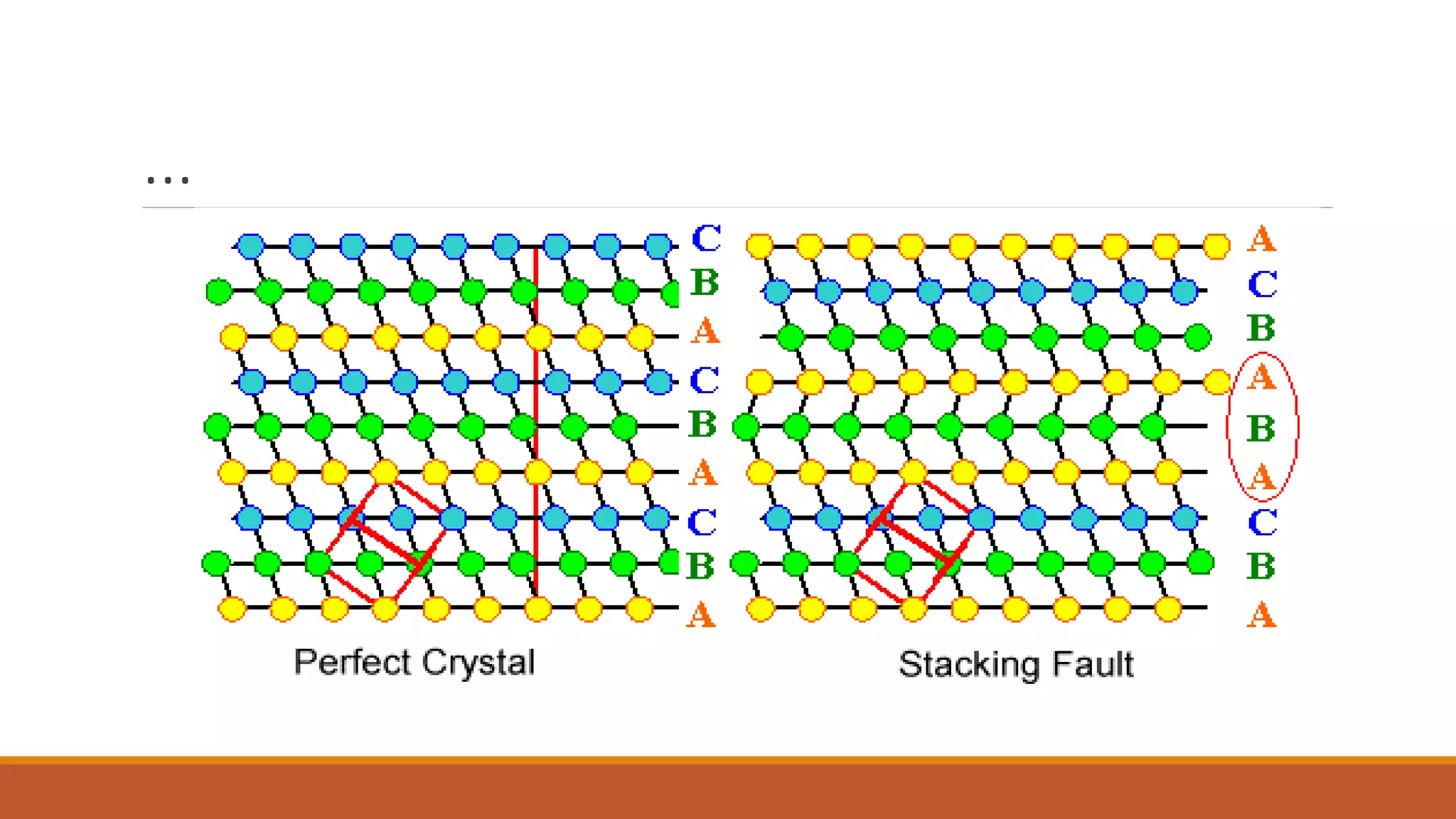
The document discusses crystal defects, categorizing them into point, line, surface, and volume defects. It explores various types of point defects, such as impurity, stoichiometric, and non-stoichiometric defects, along with examples like Frenkel and Schottky defects. Additionally, it outlines line defects (dislocations) and surface defects, including external and internal imperfections, highlighting their impact on the properties of crystals.