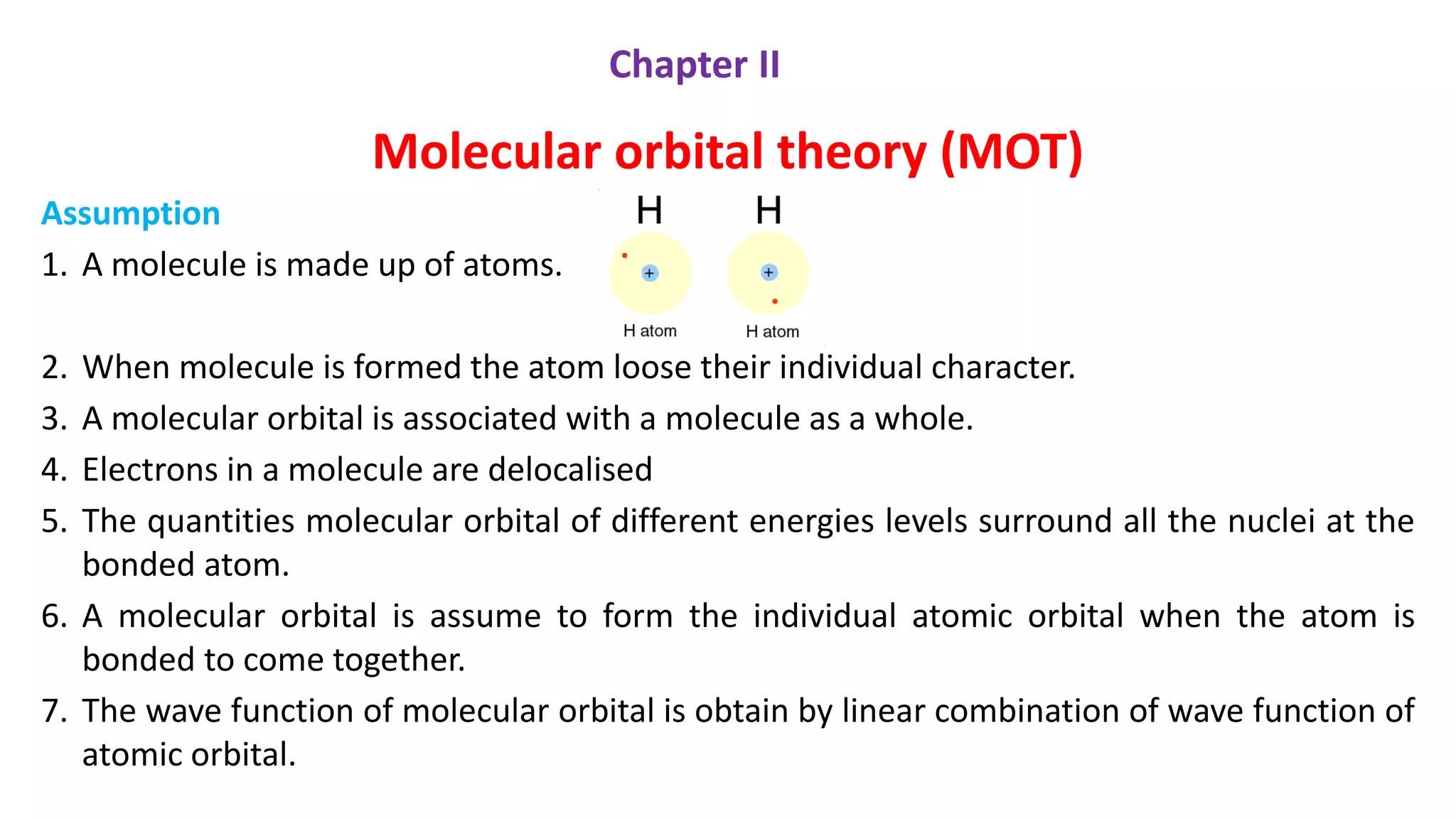
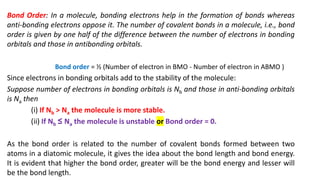
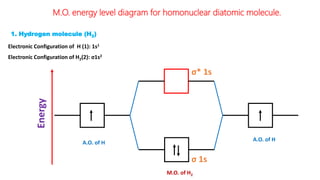
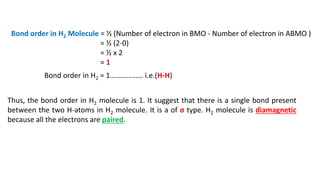
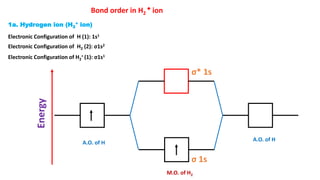
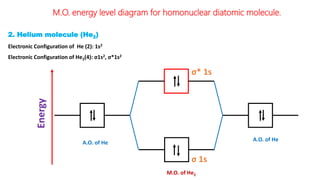
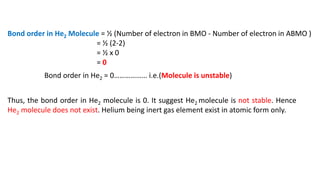
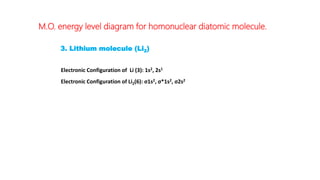
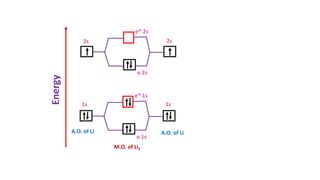
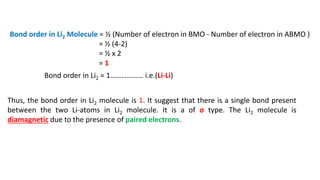
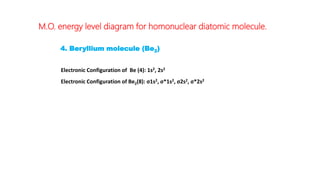
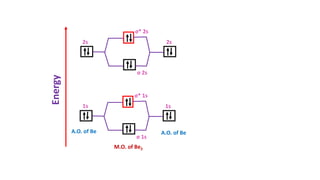
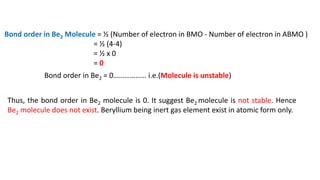
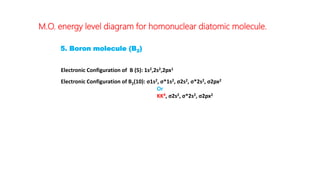
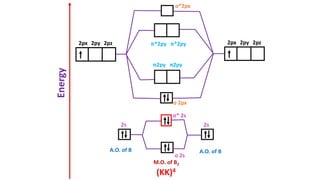
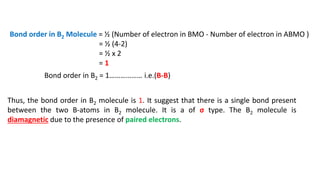
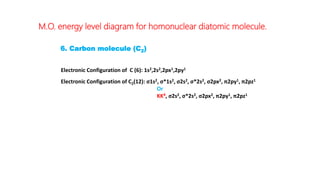
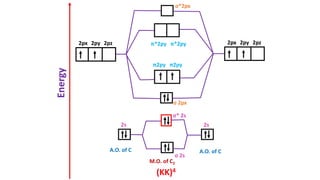
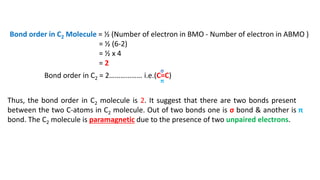
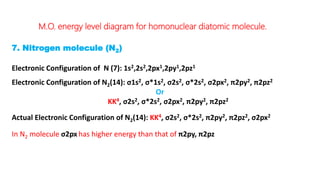
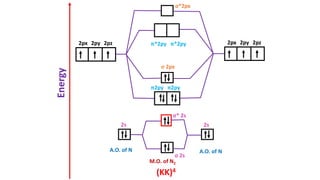
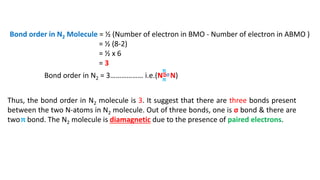
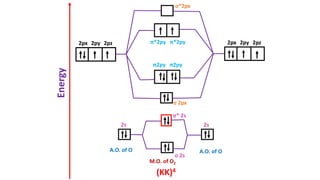
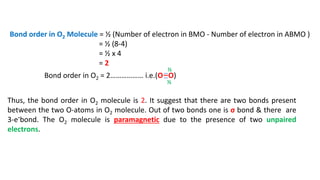
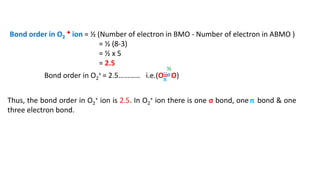
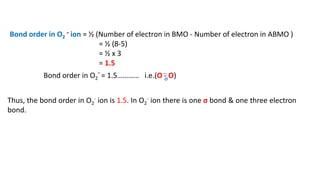
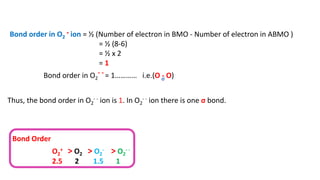
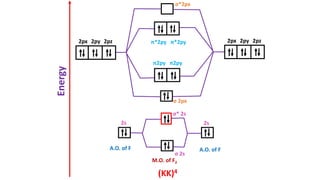
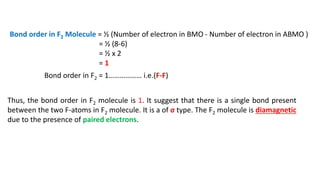
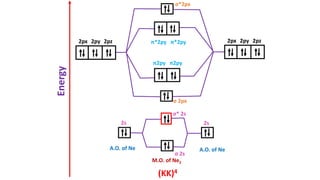
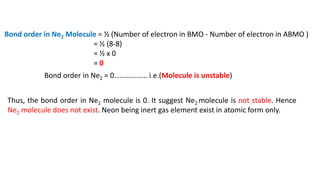
The document explains molecular orbital theory (MOT) and its assumptions regarding the formation of molecules from atoms, the delocalization of electrons, and the concept of bond order, which relates to molecular stability. It details the bonding characteristics, electron configurations, and bond orders of various homonuclear diatomic molecules including H2, He2, Li2, Be2, B2, C2, N2, O2, and their ions, discussing their stability and magnetic properties. The bond order is shown to influence bond energy and length, distinguishing between diamagnetic and paramagnetic species.