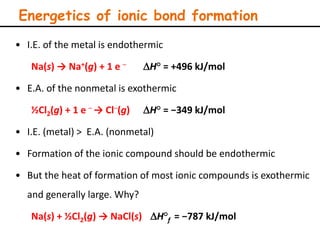
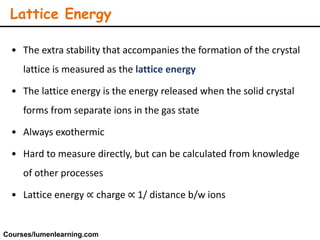
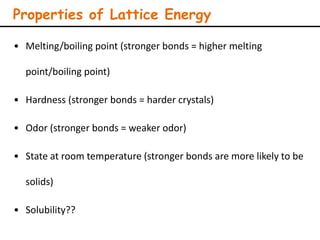
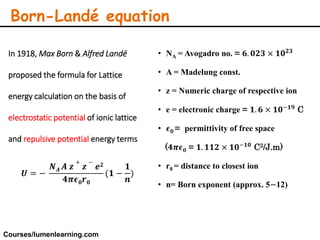
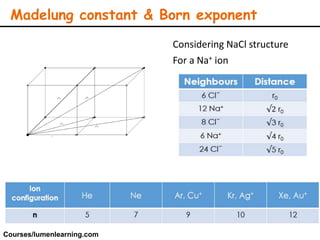
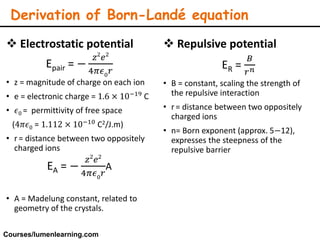
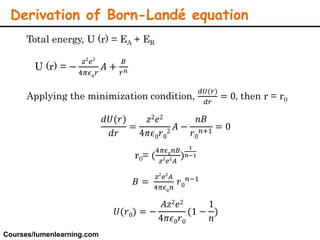
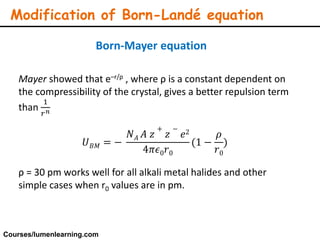
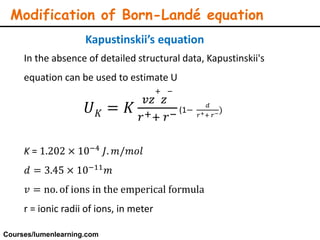
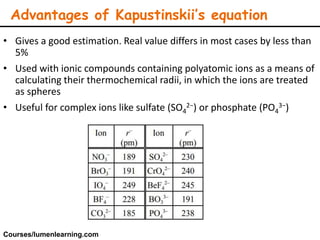
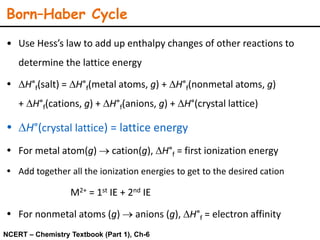
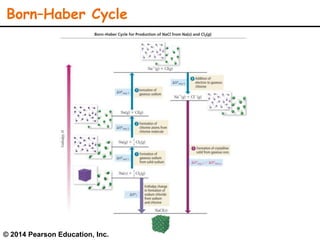
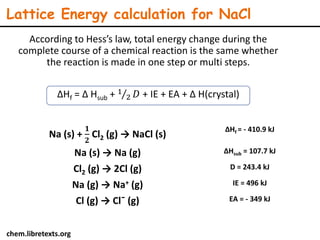
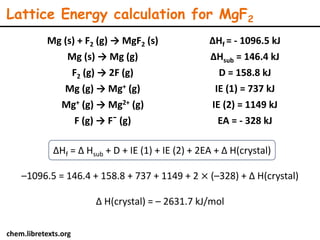
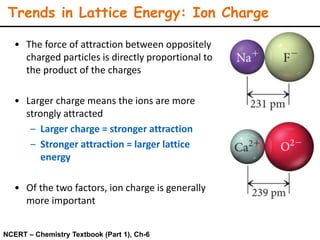
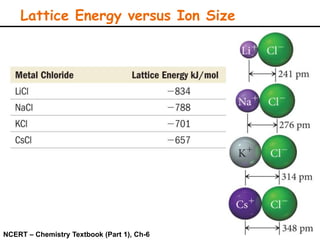
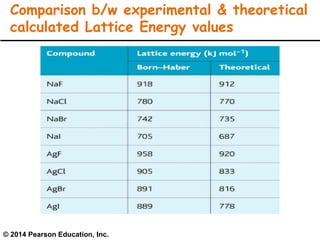
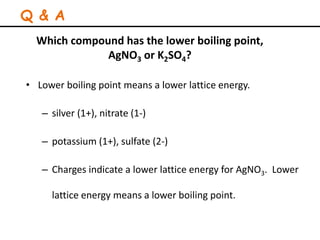
Lattice energy refers to the energy released when separate ions in the gas phase form an ionic crystal lattice. It can be calculated theoretically using the Born-Landé equation or experimentally using the Born-Haber cycle. The Born-Landé equation considers the electrostatic attraction and repulsive forces between ions, while the Born-Haber cycle uses standard enthalpy data and Hess's law. Lattice energy depends on factors like ion charge and size - higher charge or smaller ions lead to stronger electrostatic forces and higher lattice energy. Lattice energy is an important concept for understanding the properties and stability of ionic compounds.