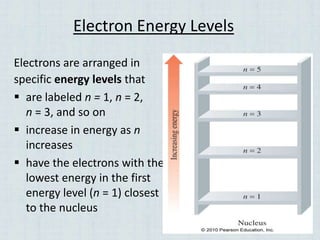
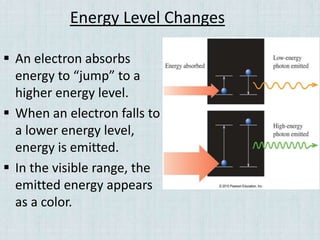
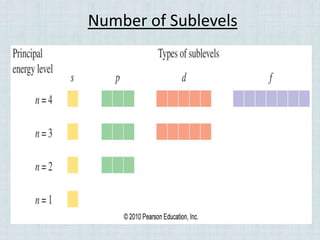
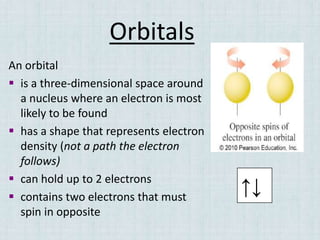
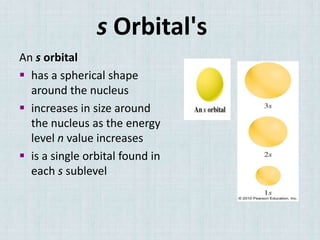
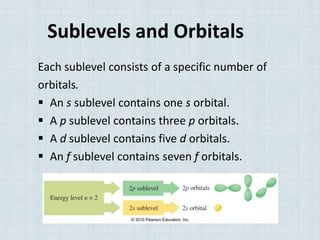
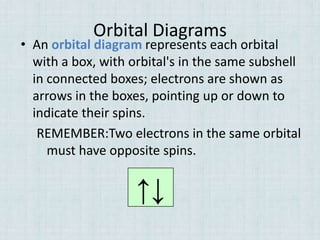
Electrons occupy specific energy levels labeled n=1, 2, 3 etc, with increasing energy as n increases. Within each level are sublevels that further organize the electrons. The s sublevel has the lowest energy, followed by p, d and f sublevels. Orbitals represent the spaces electrons occupy, with different shapes for s, p, d and f orbitals. Orbital diagrams visually depict the arrangement of electrons in orbitals and energy levels.