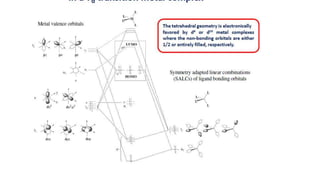
This document discusses molecular orbital theory and its application to octahedral, tetrahedral, and square planar transition metal complexes. It explains that molecular orbitals are formed from the combination of atomic orbitals on the metal center and surrounding ligands. These molecular orbitals can be bonding, antibonding, or nonbonding. For octahedral complexes, the dz2 and dx2-y2 orbitals form bonding and antibonding orbitals with ligand orbitals. The dxy, dyz, and dxz orbitals remain nonbonding. Tetrahedral complexes have fewer symmetries but the metal orbitals can still be classified. Antibonding orbitals are higher in energy than bonding orbitals due to rep