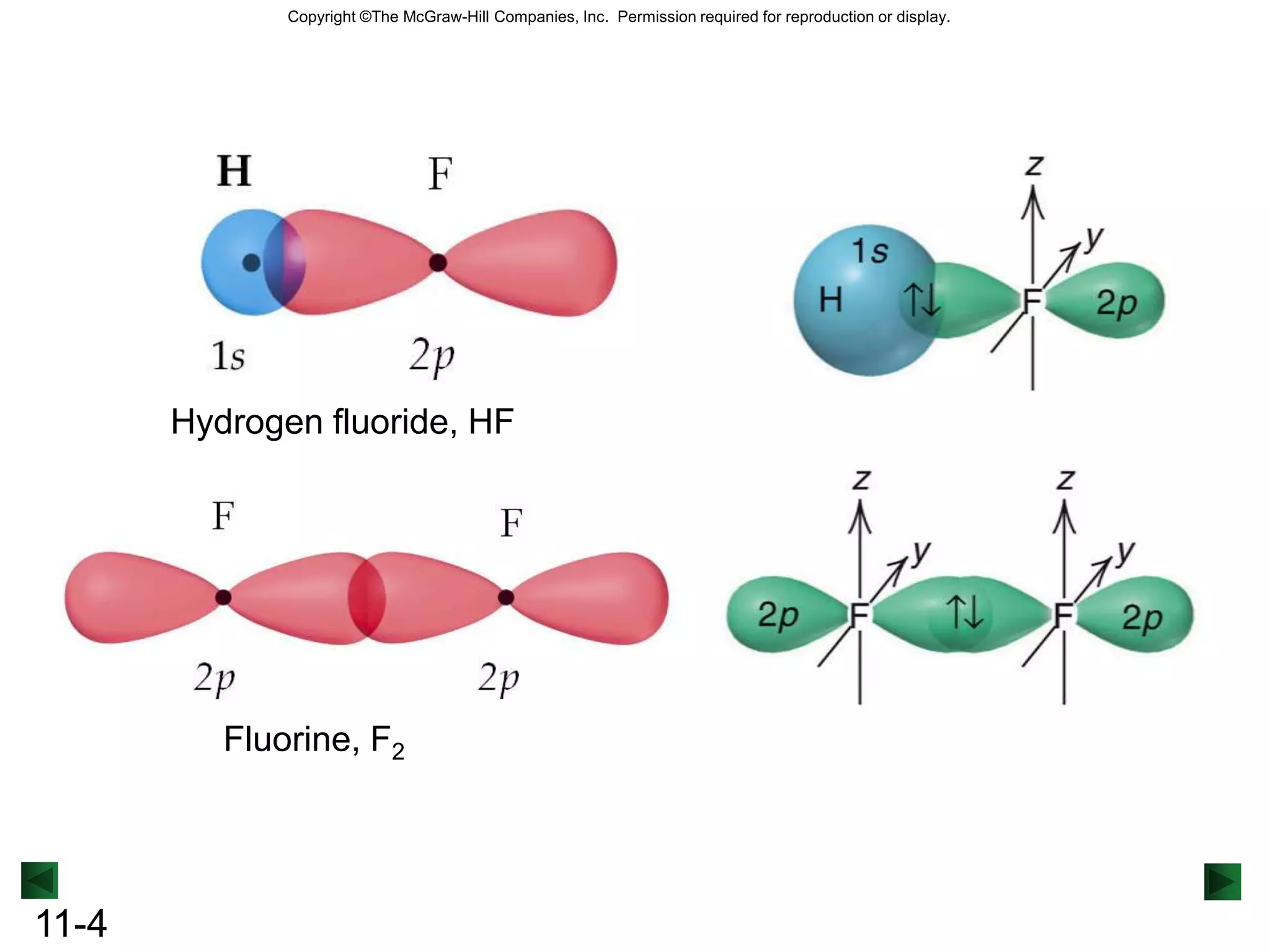
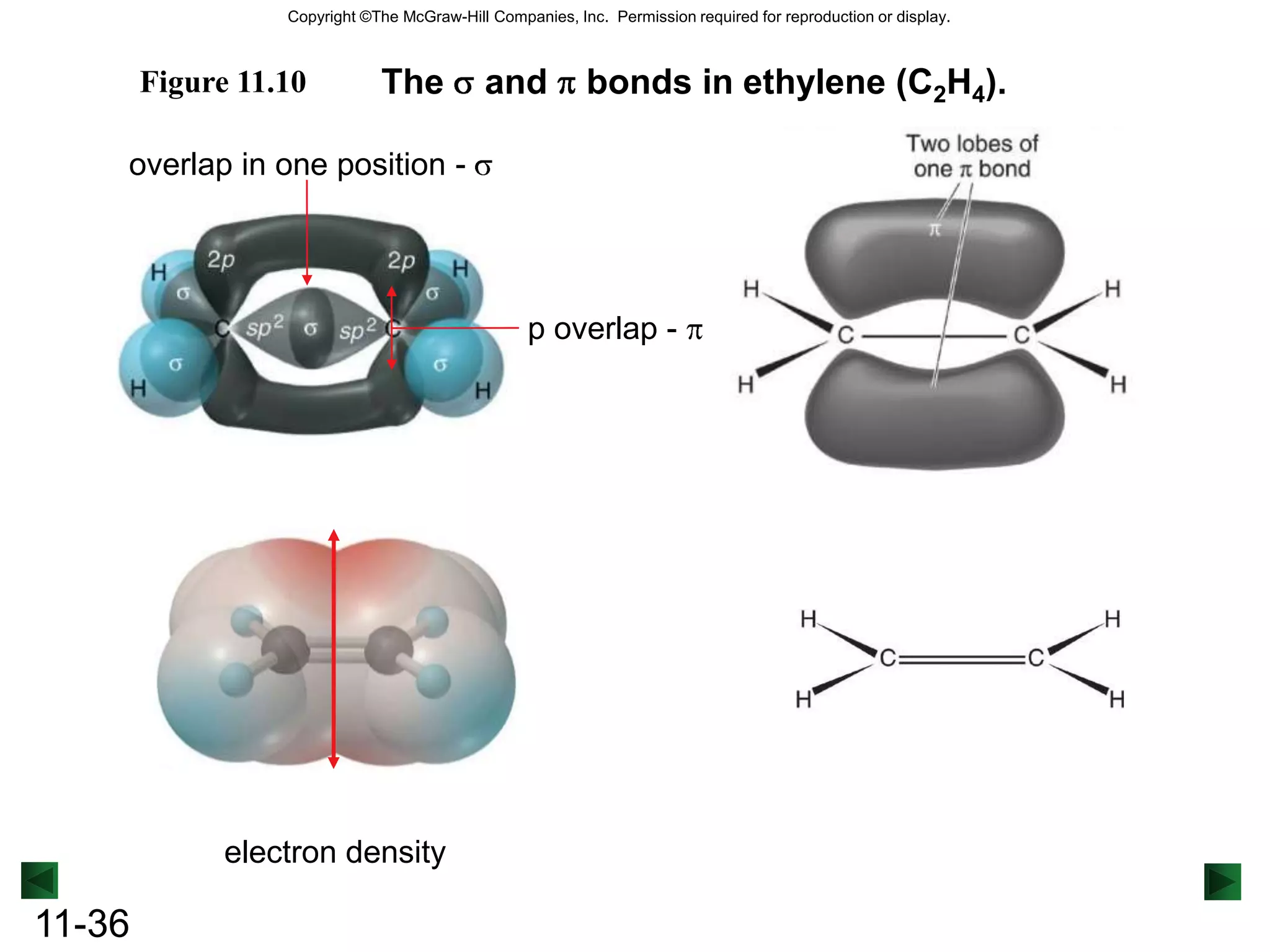
The document discusses theories of covalent bonding including valence shell electron pair repulsion theory, valence bond theory, orbital hybridization, and molecular orbital theory. It focuses on how orbitals overlap to form bonds between atoms and the different types of hybrid orbitals that can form based on electron domain geometry. Examples are provided to illustrate sp, sp2, sp3, and other hybrid orbitals as well as sigma and pi bonding including delocalized pi bonding in benzene.