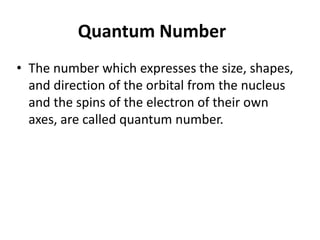
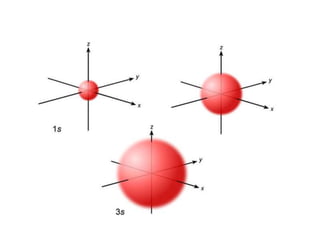
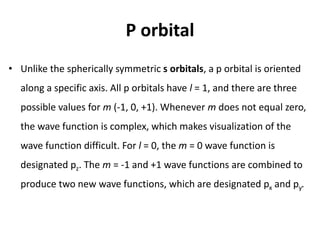
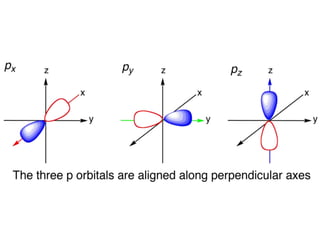
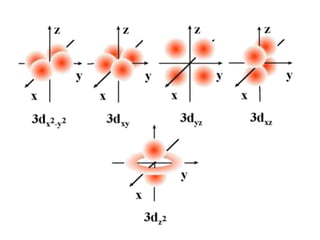
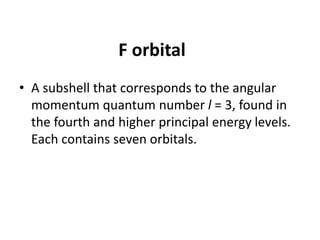
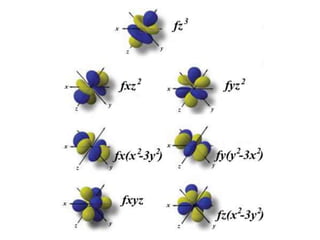
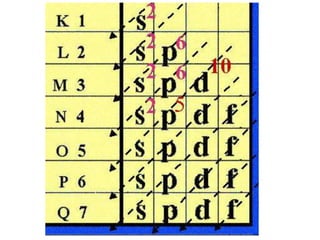
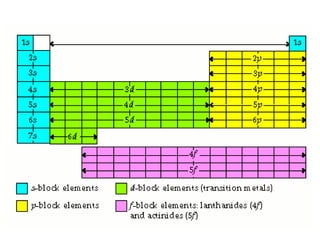
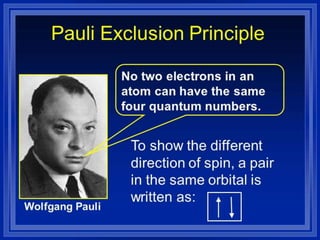
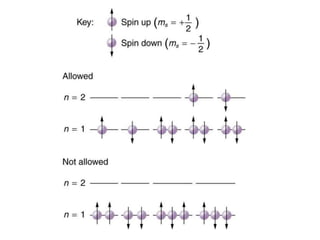
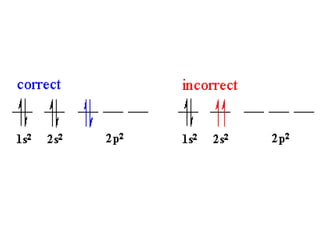
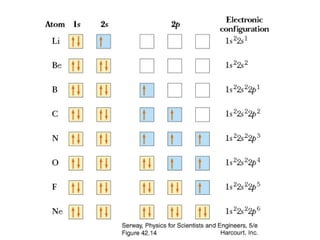
An atomic orbital is defined as the region around the nucleus where an electron is most likely to be found. There are four quantum numbers that describe the orbitals: principal (n), angular momentum (l), magnetic (m), and spin (s). The principal quantum number indicates the electron shell and average distance from the nucleus. The angular momentum number determines the shape of the orbital. The magnetic number indicates the direction of the orbital. The spin number represents the electron's clockwise or counterclockwise spin. Common atomic orbitals include s, p, d, and f orbitals, which have different shapes and orientations determined by their quantum numbers.