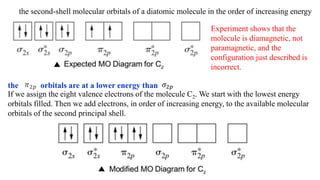
Molecular orbital theory is an advanced quantum mechanical model explaining bonding in molecules using atomic orbitals and the Heisenberg uncertainty principle. It describes the formation of bonding and antibonding molecular orbitals through constructive and destructive interference of wave functions, determining electron probability density and bond order. Key concepts include the filling order of orbitals, Pauli exclusion principle, and Hund's rule, alongside examples of molecular configurations for various diatomic molecules.







![Electron probability or electron charge density
The electron probability or electron charge density in the 𝜎1𝑠orbital is
1𝑠 𝐴 + 1𝑠 𝐵
2
The square has an extra term [2 x 1𝑠 𝐴 1𝑠 𝐵] which is the extra charge density between the
nuclei.
A high electron charge density between atomic nuclei reduces repulsions between the
positively charged nuclei and promotes a strong bond. This bonding molecular orbital,
designated 𝜎1𝑠, is at a s lower energy than the 1s atomic orbitals.
The electron charge density in the molecular orbital formed by destructive interference of
the 2, 𝜎1𝑠
∗
orbital is reduced 1𝑠 𝐴 − 1𝑠 𝐵
2
The square has minus term [2 x 1𝑠 𝐴 1𝑠 𝐵] which reduces the charge density between the
nuclei.
A low electron charge density between atomic nuclei promotes repulsions between the
positively charged nuclei. This bonding molecular orbital, designated 𝜎 ∗1𝑠, is at the higher
energy than the 1s atomic orbitals.](https://image.slidesharecdn.com/molecularorbitaltheory113-200331212244/85/Molecular-orbital-theory-11-3-8-320.jpg)