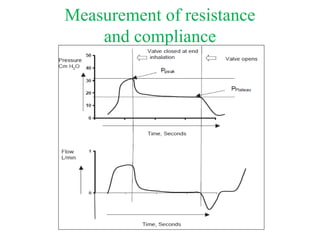
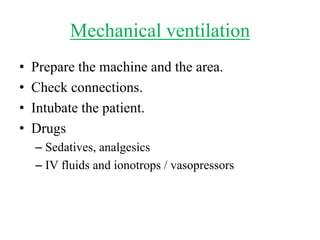
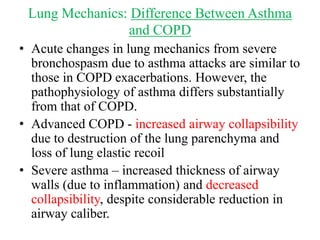
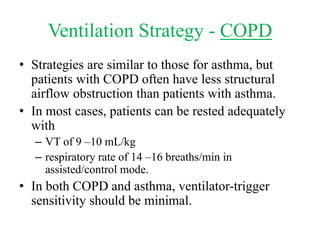
This document discusses mechanical ventilation for patients with obstructive airway diseases like COPD. Some key points:
- Non-invasive ventilation (NIV) should be considered within 60 minutes of hospital arrival for COPD patients with respiratory acidosis, as NIV can reduce intubation and mortality rates.
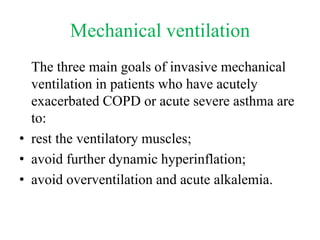
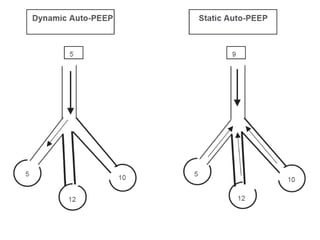
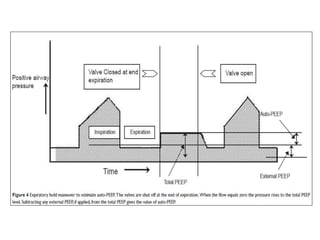
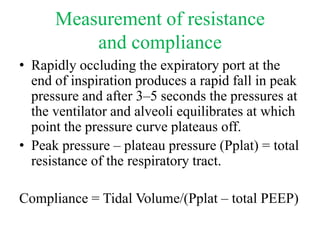
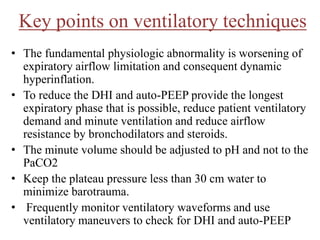
- Invasive mechanical ventilation aims to rest respiratory muscles, avoid dynamic hyperinflation, and prevent overventilation. Dynamic hyperinflation can increase work of breathing and compromise cardiac function.
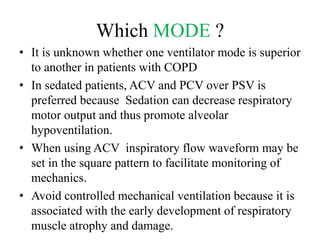
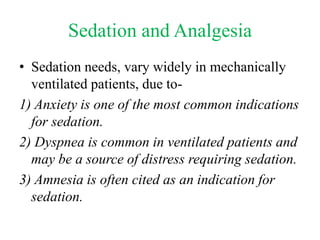
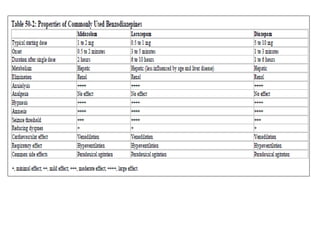
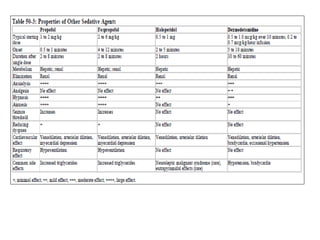
- Ventilation strategies differ between asthma and COPD but generally use small tidal volumes, high inspiratory flows, and respiratory rates to minimize hyperinflation. Sedation and analgesia are also important to control distress and pain