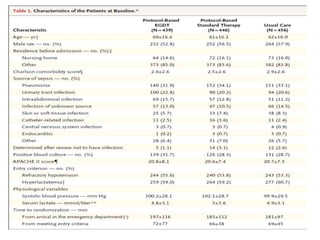
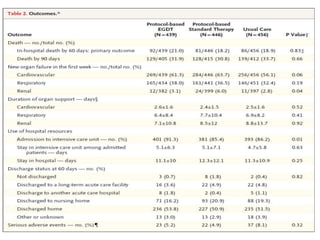
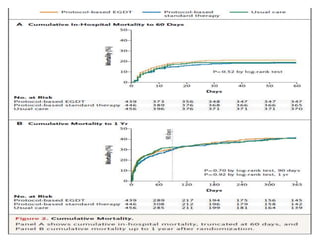
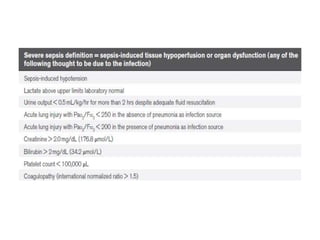
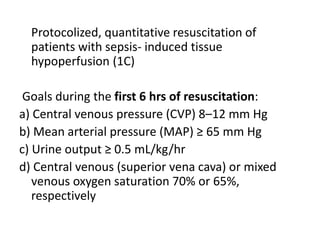
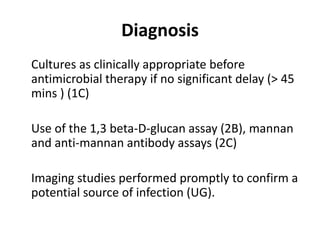
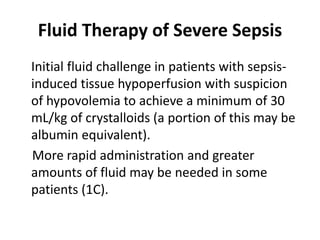
The ProCESS trial found no significant difference in mortality between patients receiving protocol-based early goal-directed therapy (EGDT), protocol-based standard therapy, or usual care for early septic shock. While the EGDT group received fluid resuscitation more consistently, there were no differences in outcomes like mortality, organ failure, or length of stay. This prompted review by the Surviving Sepsis Campaign. They concluded that early diagnosis and treatment remain important. ProCESS did not assess patients with severe sepsis without shock. It also showed improved usual care since 2001, with mortality dropping from 46.5% to 18%, so key bundles should still be followed.