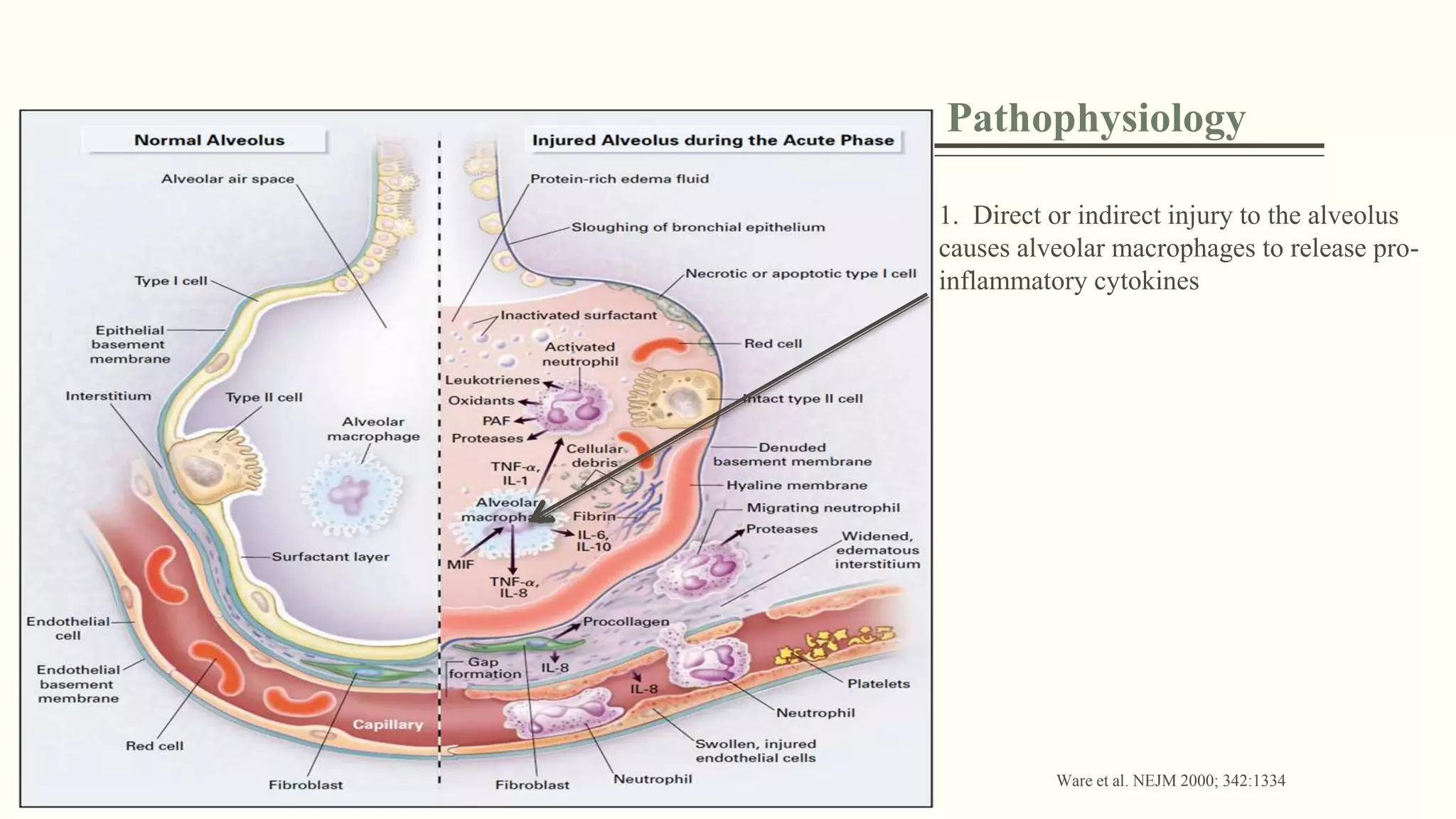
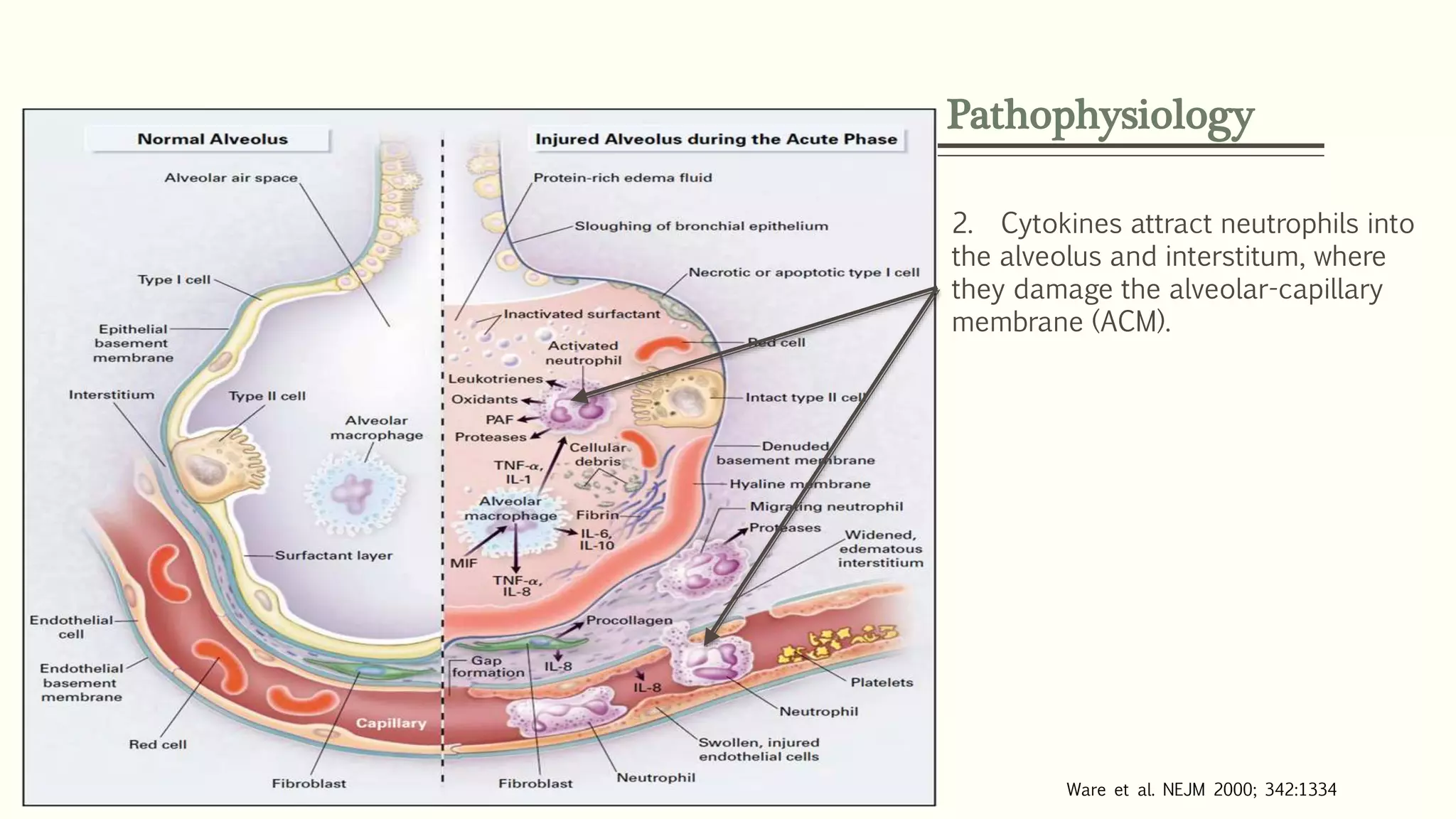
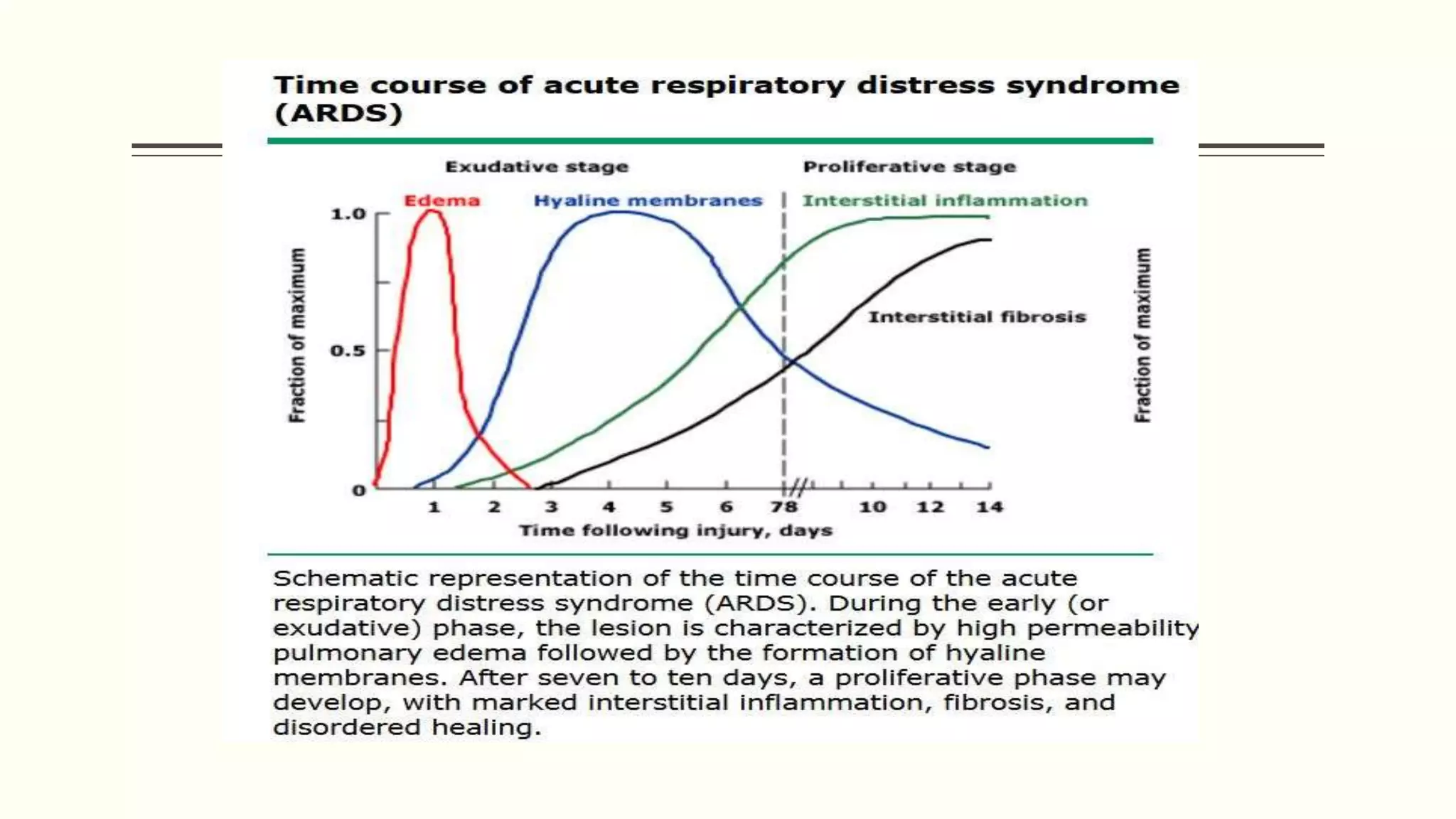
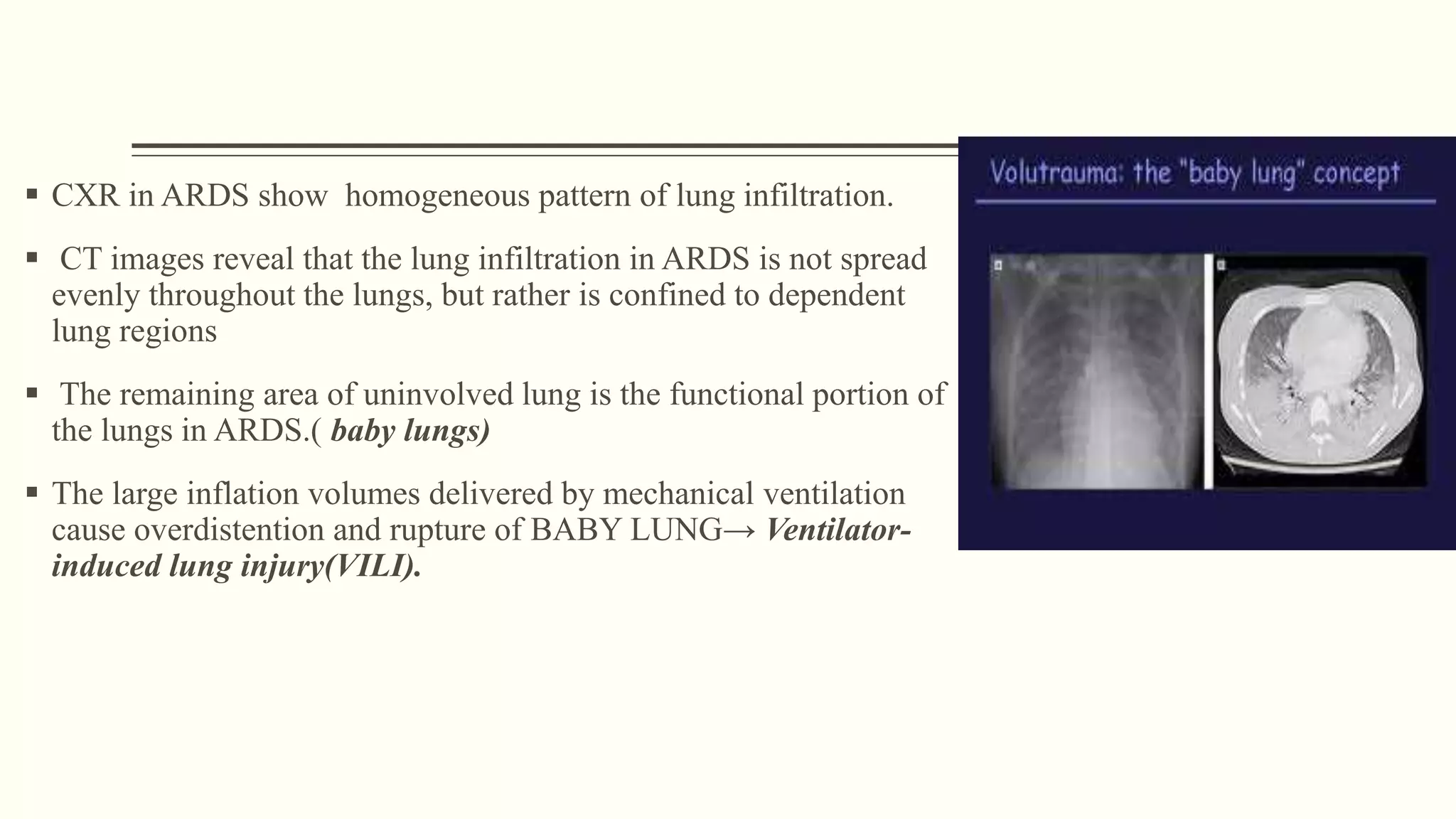
1) ARDS is characterized by hypoxemia, bilateral lung infiltrates, and respiratory failure not fully explained by cardiac failure. The Berlin definition classifies ARDS as mild, moderate, or severe based on oxygenation levels.
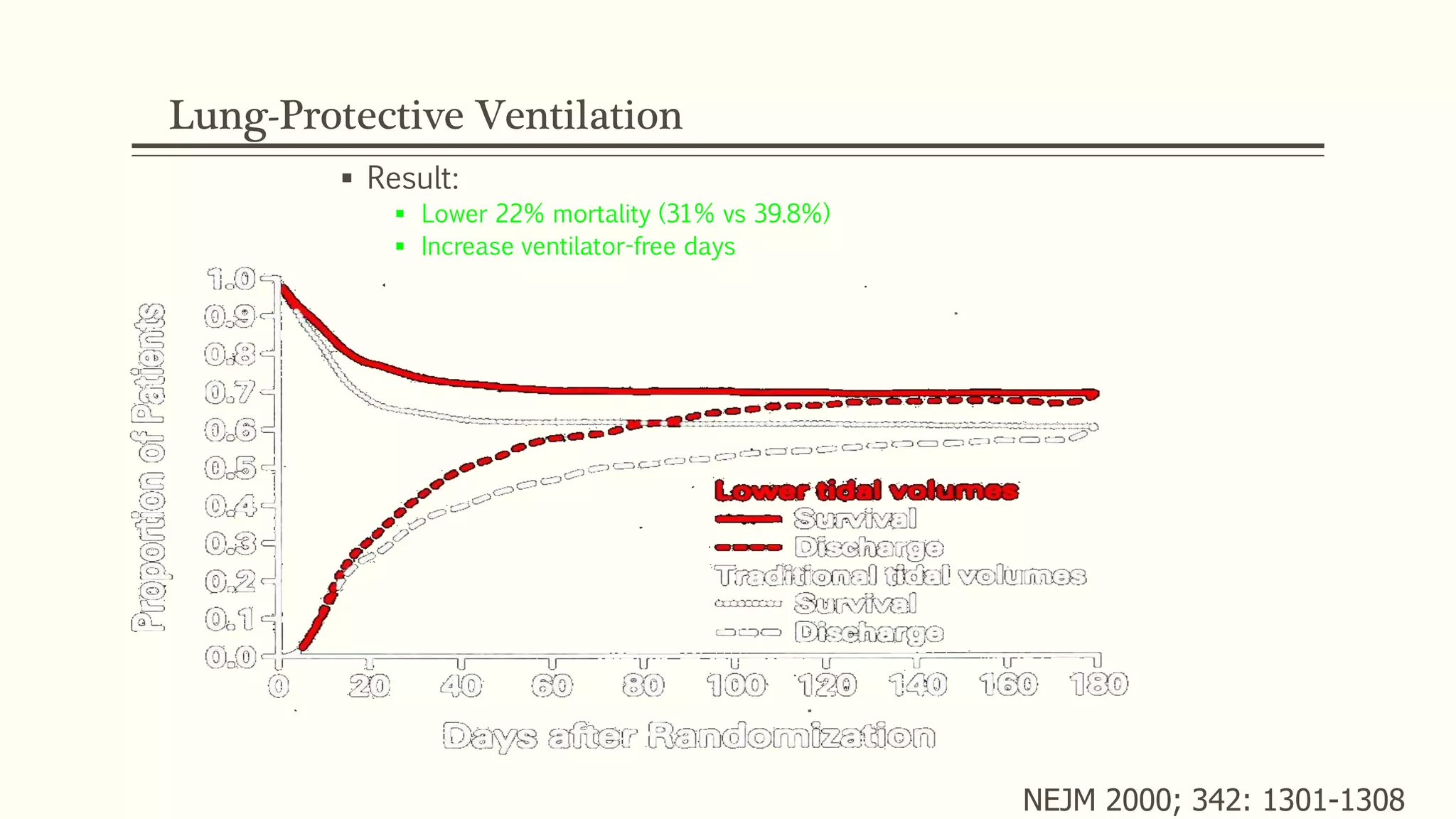
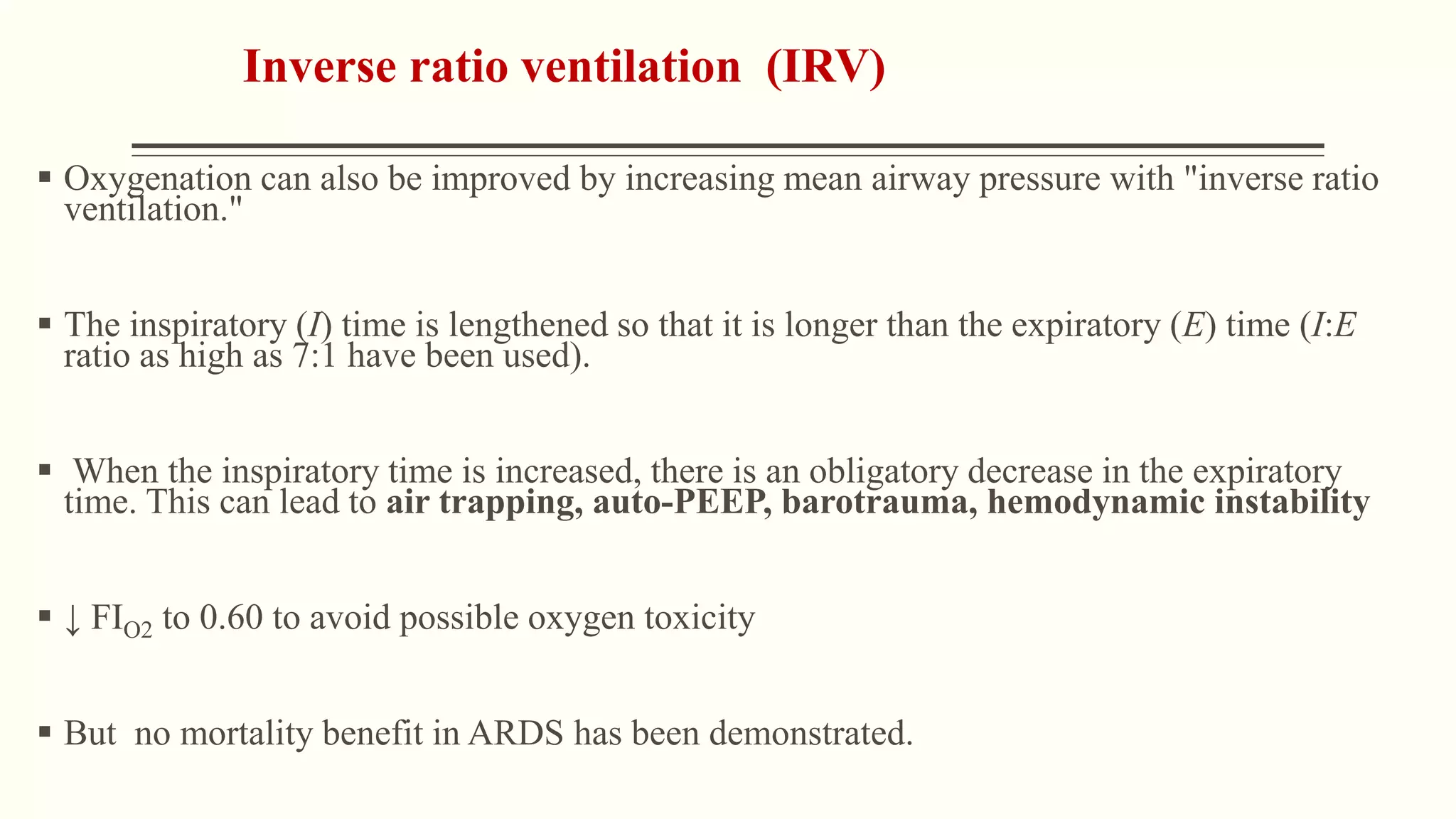
2) Management of ARDS focuses on treating underlying causes, preventing complications, and using ventilator strategies like low tidal volume ventilation to prevent ventilator-induced lung injury.
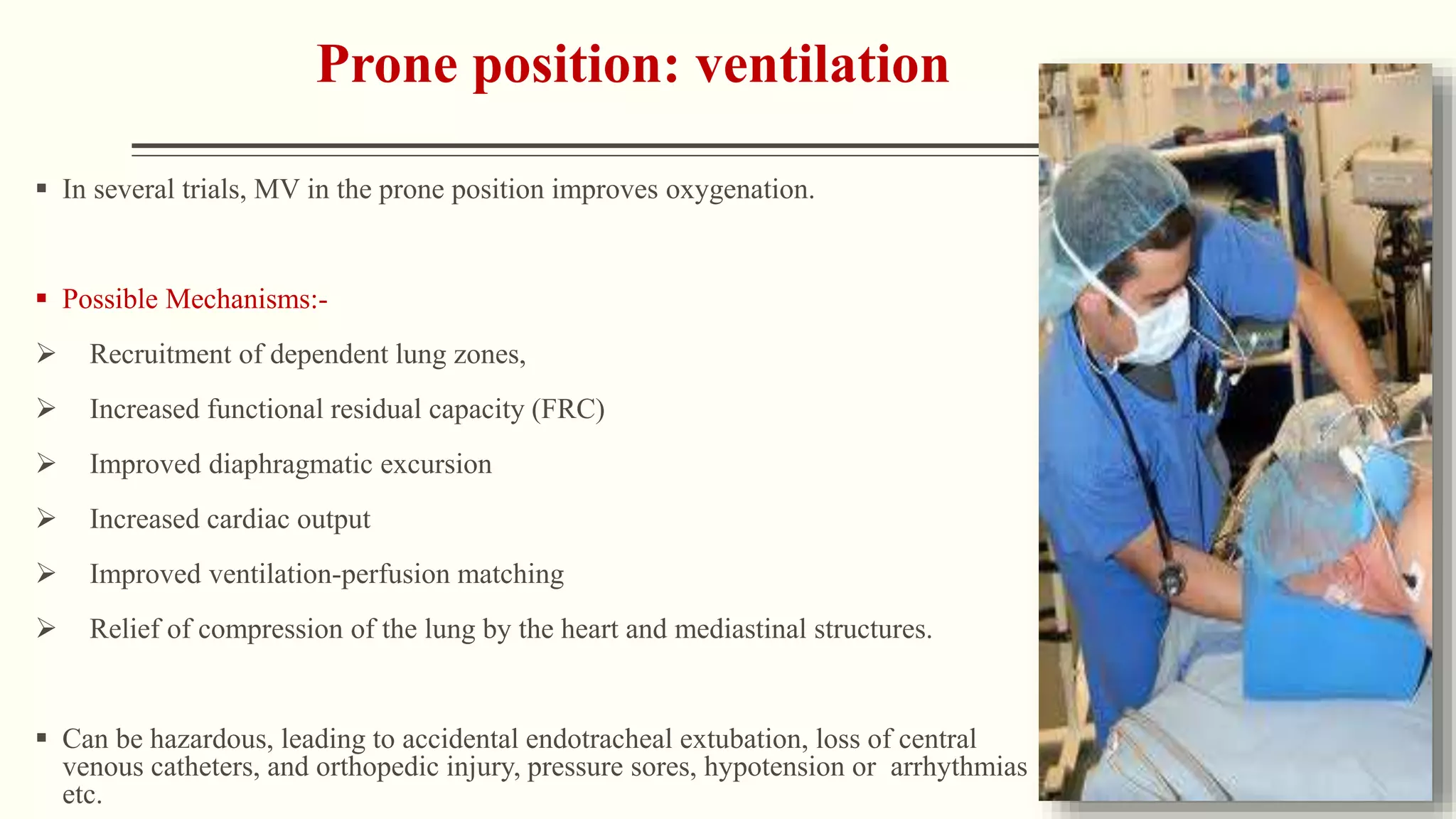
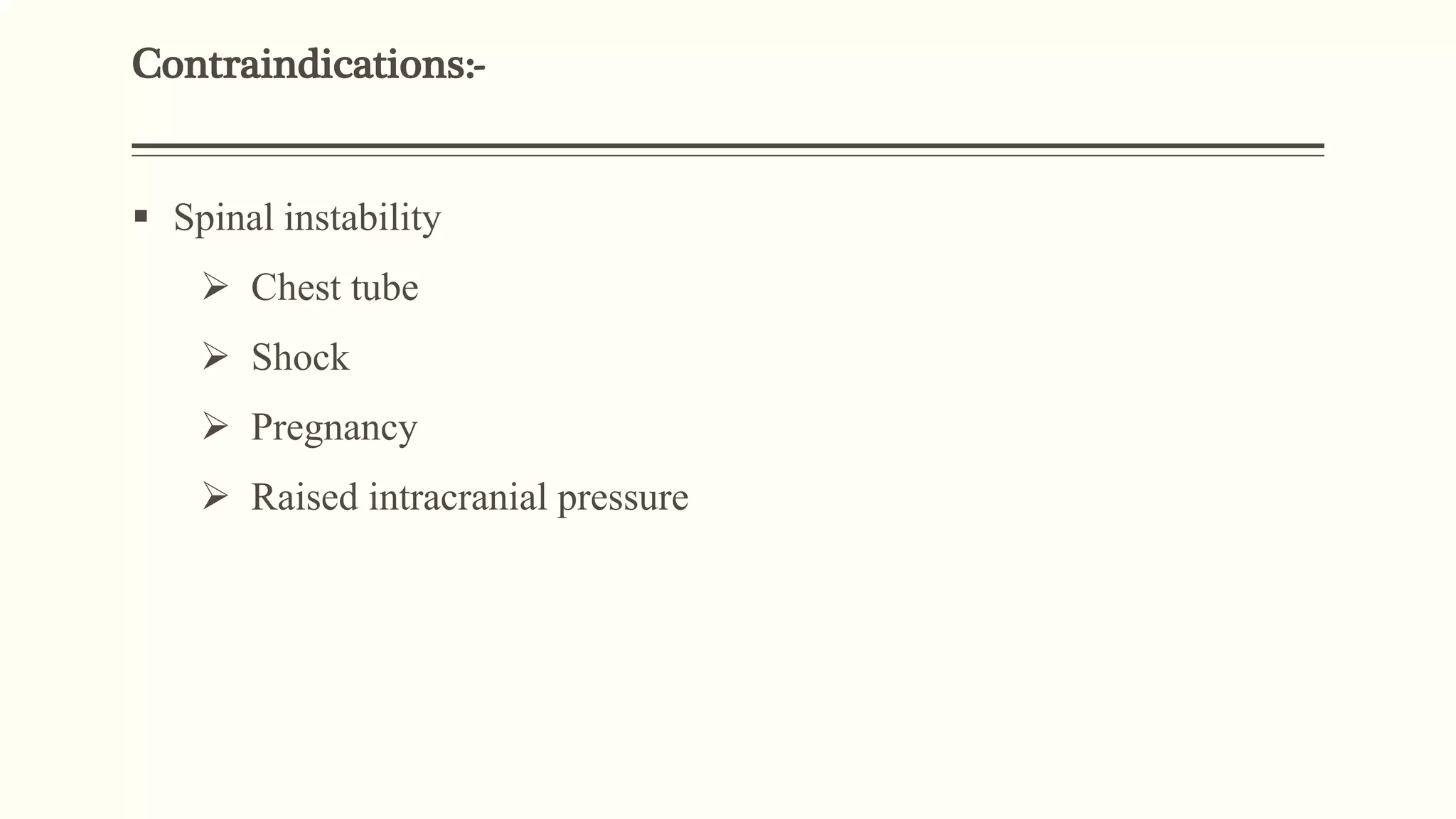
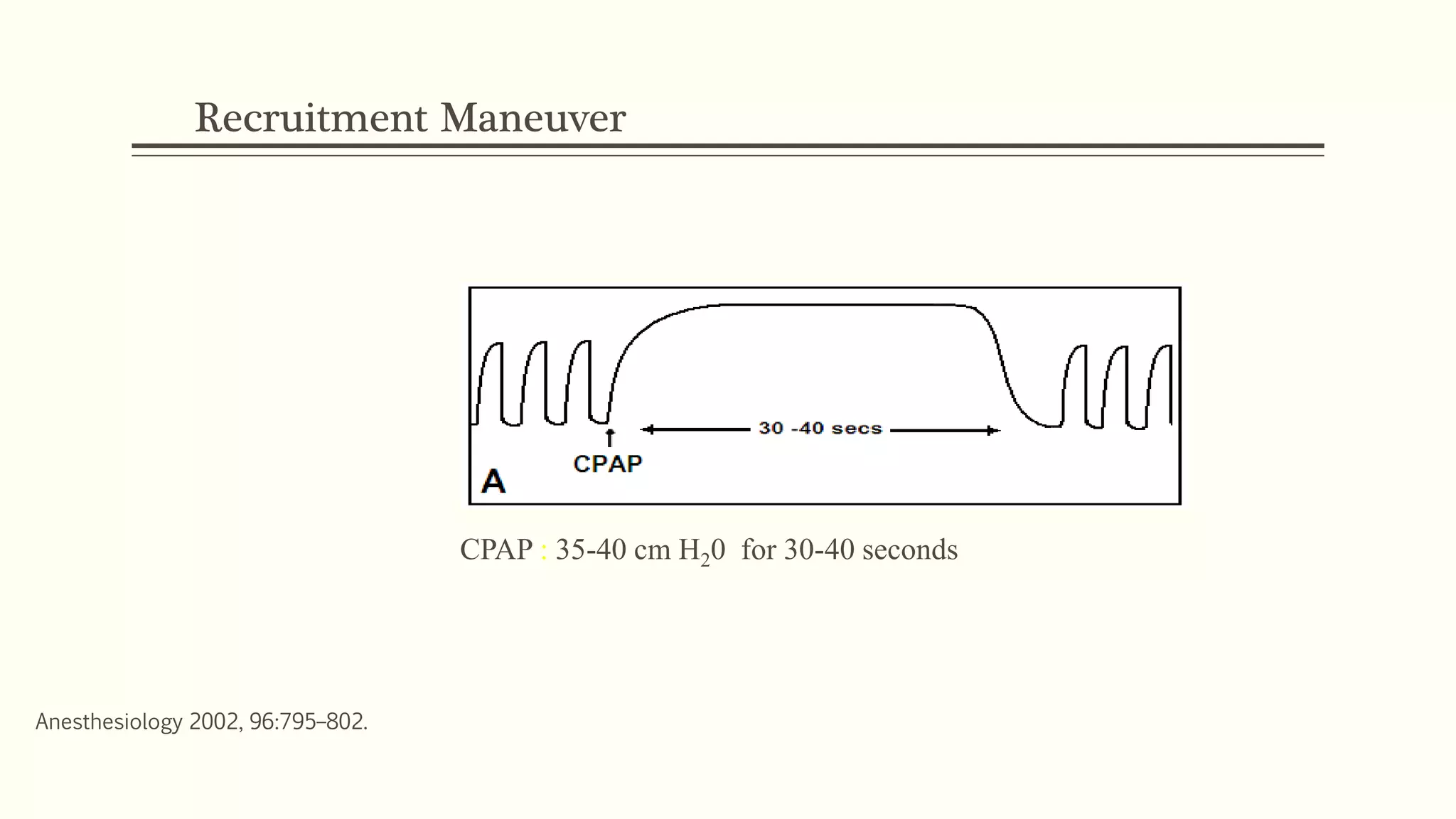
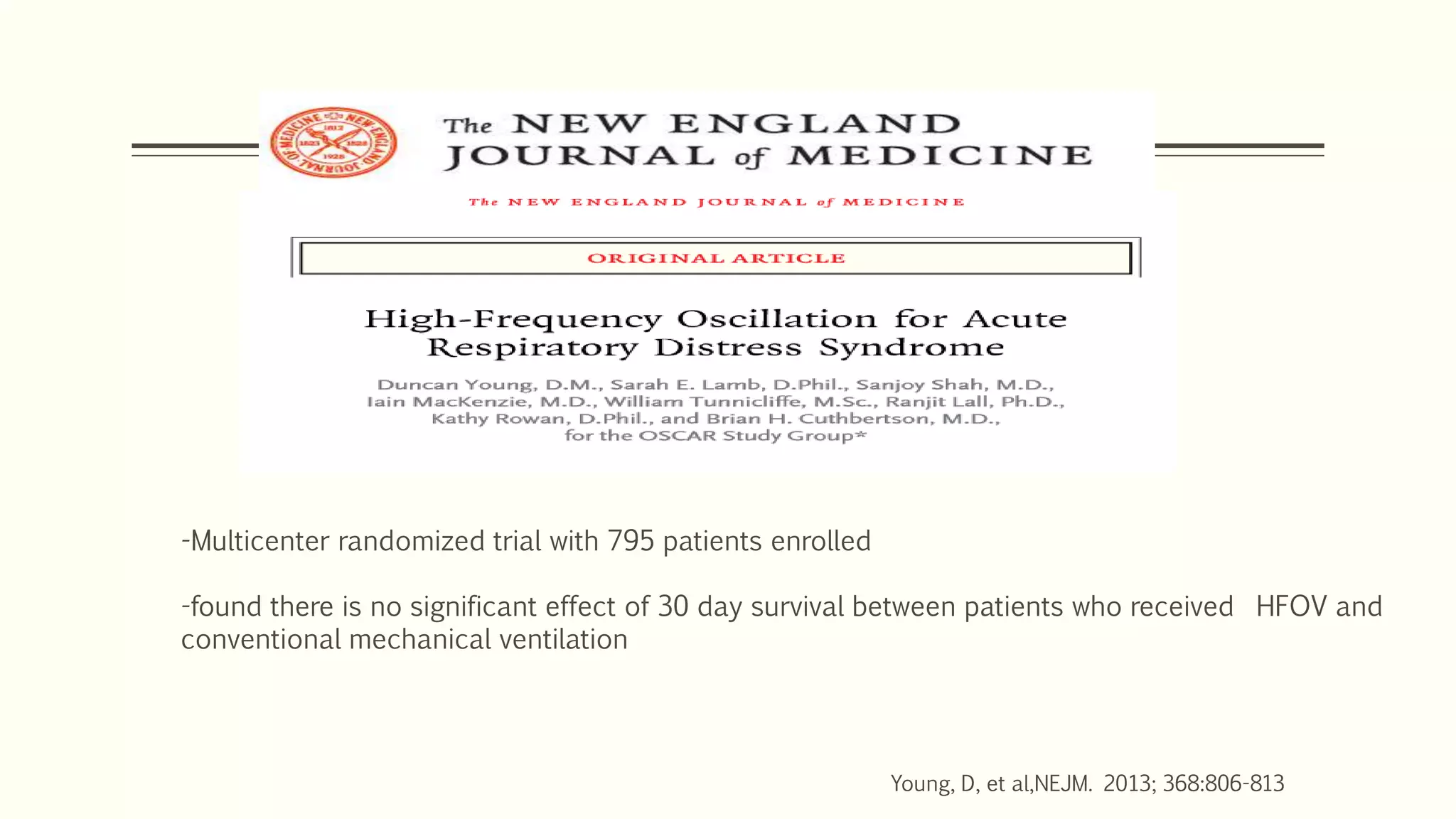
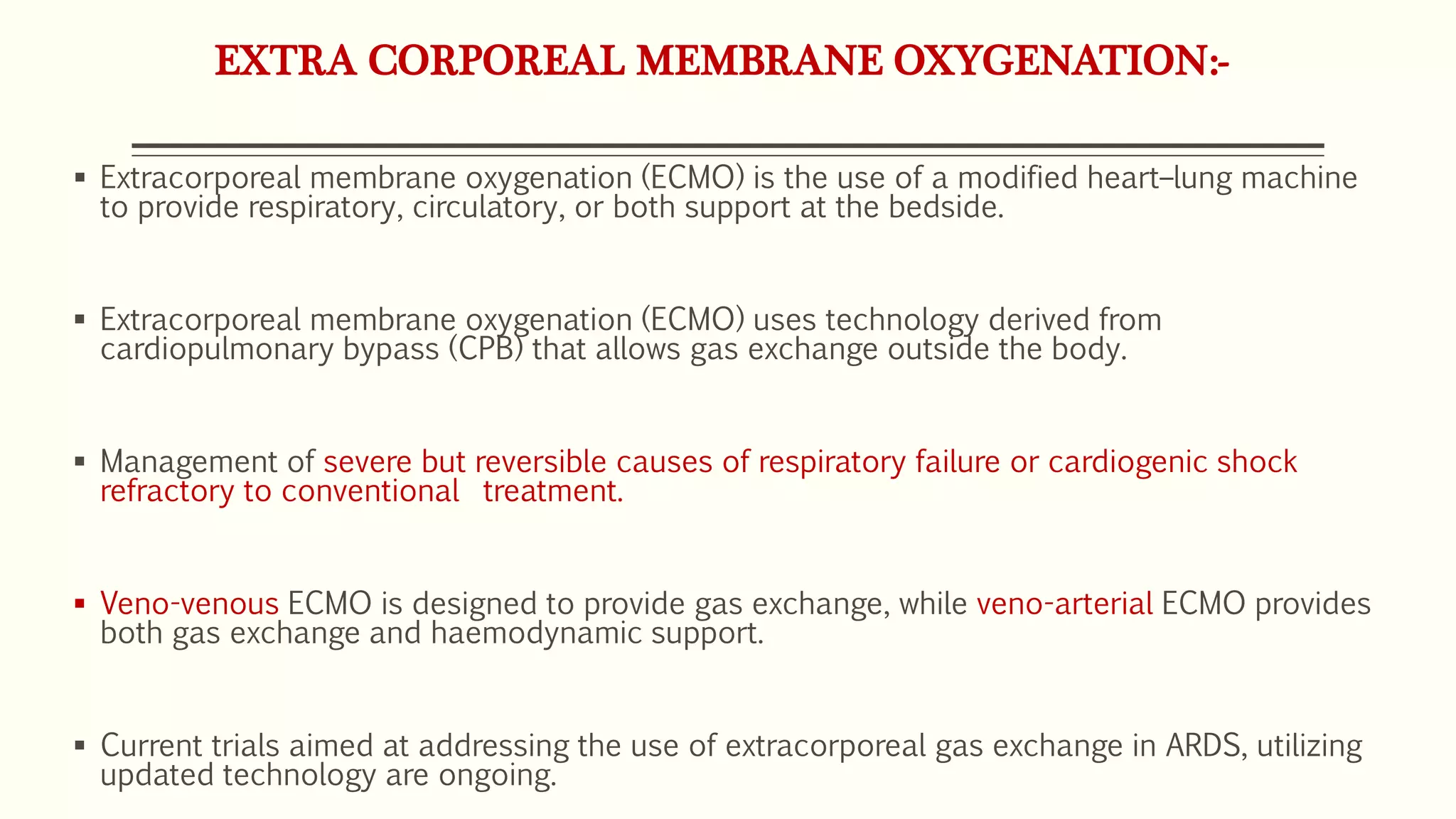
3) Other ventilator strategies discussed include prone positioning, neuromuscular blockade, recruitment maneuvers, and extracorporeal membrane oxygenation for severe cases, though evidence on benefits is mixed.


![ARDS definition - AECC 1994
1. Acute onset of hypoxemia (arterial partial pressure of oxygen to fraction of inspired oxygen
[PaO2/FIO2] ≤ 200 mm Hg)
2. Bilateral infiltrates on frontal chest radiograph,
3. No evidence of left heart failure.
4. The presence of predisposing conditions.
(ARDS was first defined in 1994 by the American-European Consensus Conference (AECC) ).
Rawal G, Yadav S, Kumar R. Acute Respiratory Distress Syndrome: An Update and Review. J Transl Int Med. 2018;6(2):74-77. Published 2018 Jun
26. doi:10.1515/jtim-2016-0012](https://image.slidesharecdn.com/ards-190317122057/75/Ventilator-strategies-in-ARDS-3-2048.jpg)