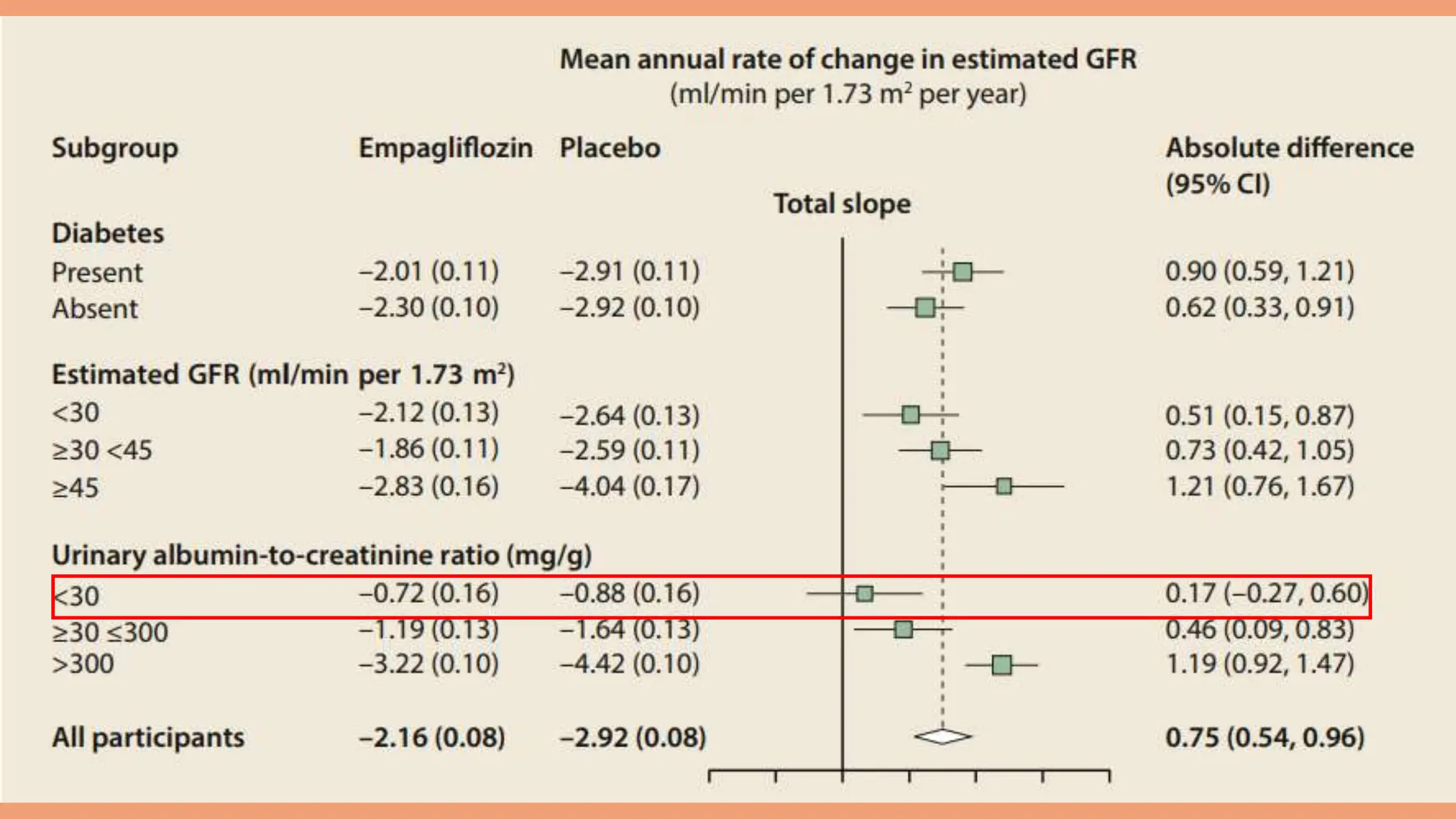
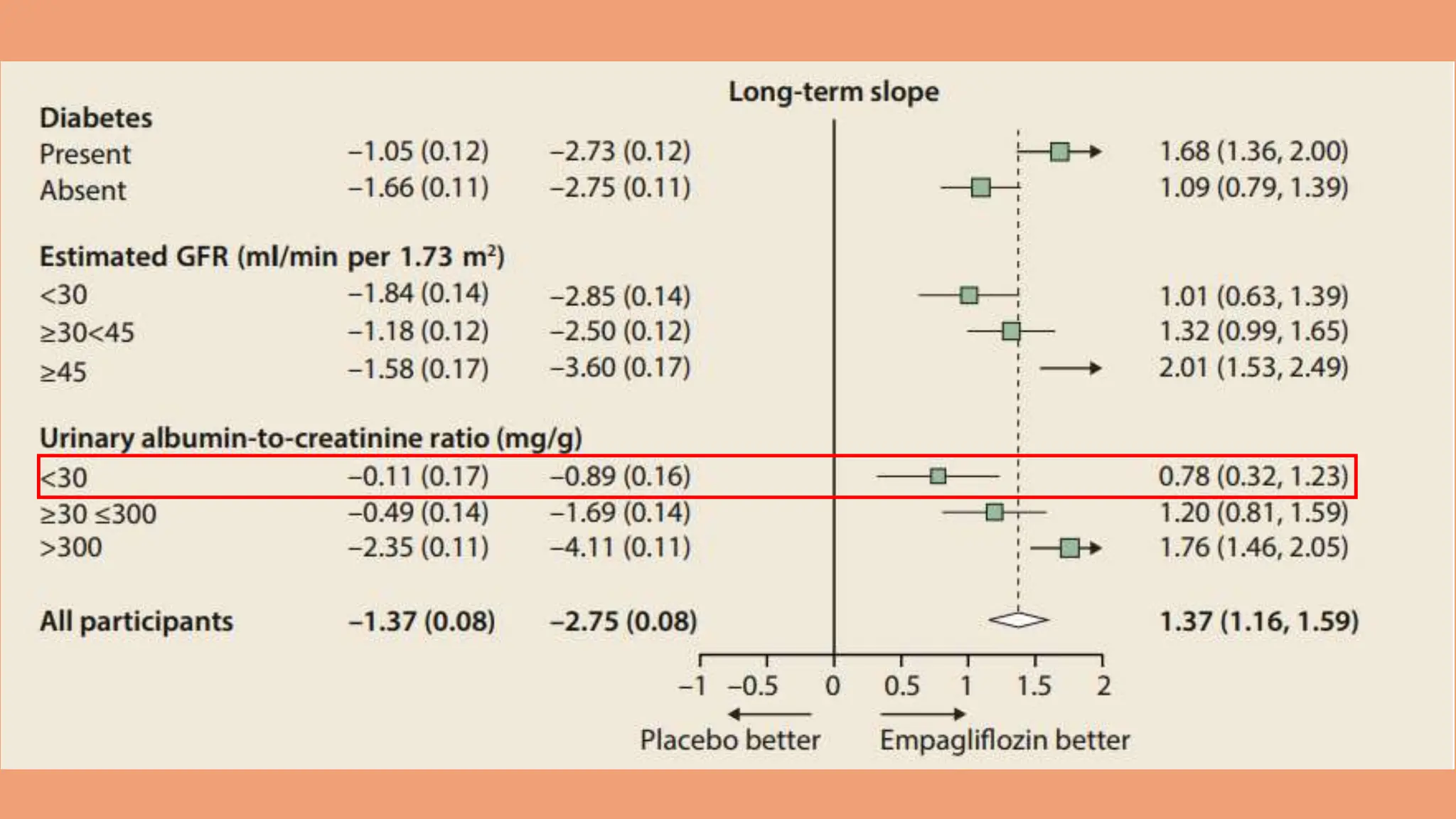
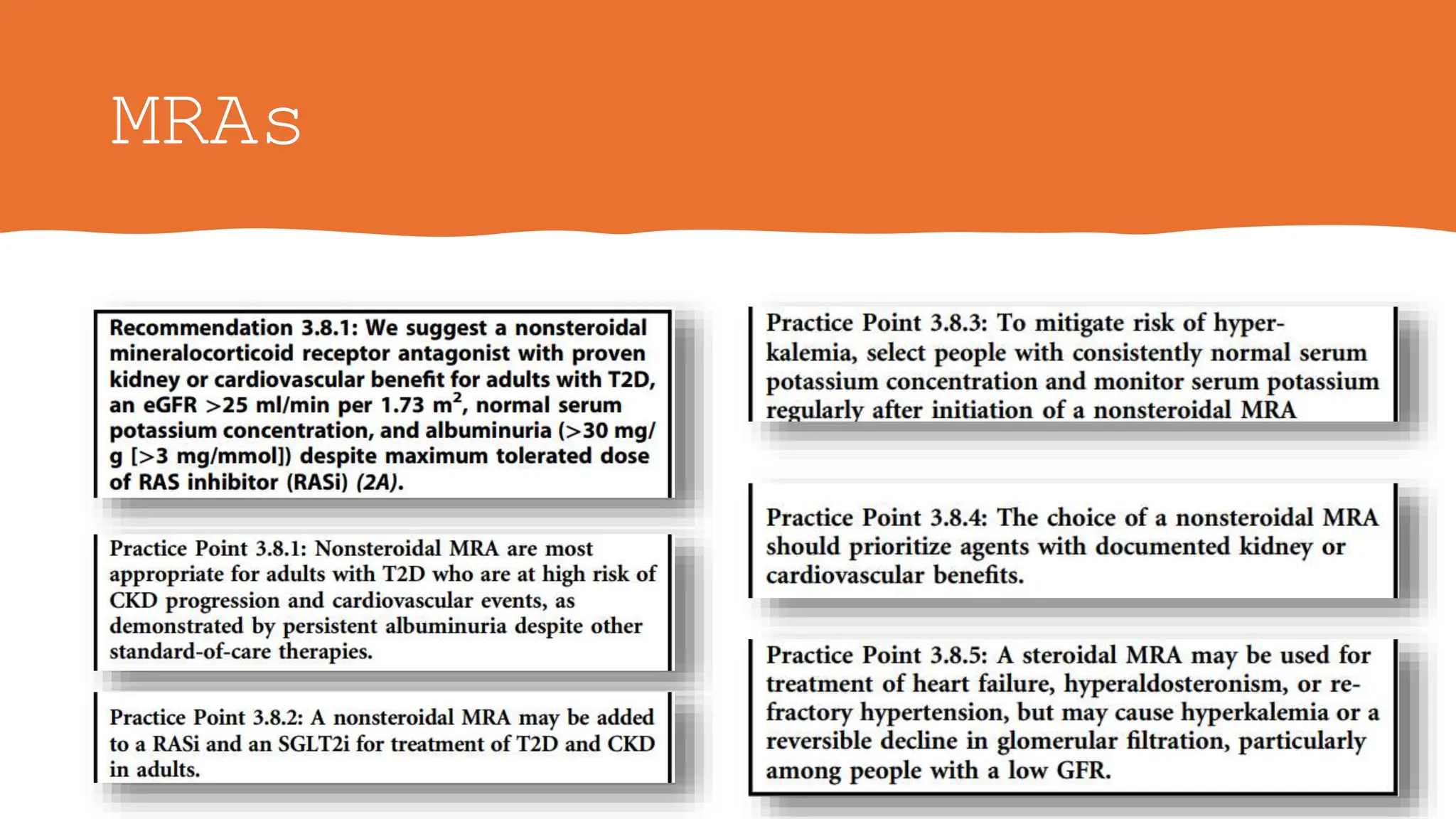
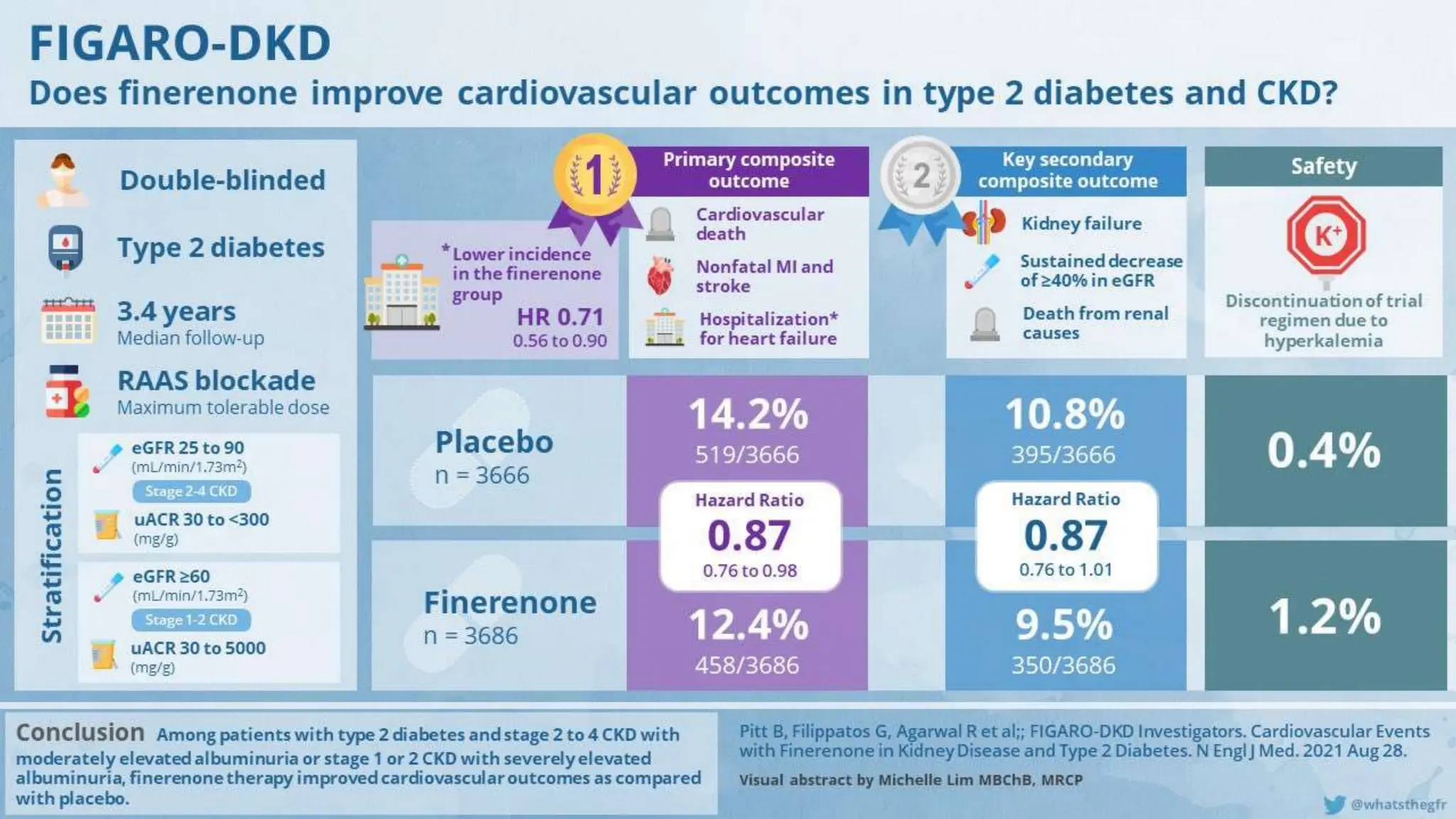
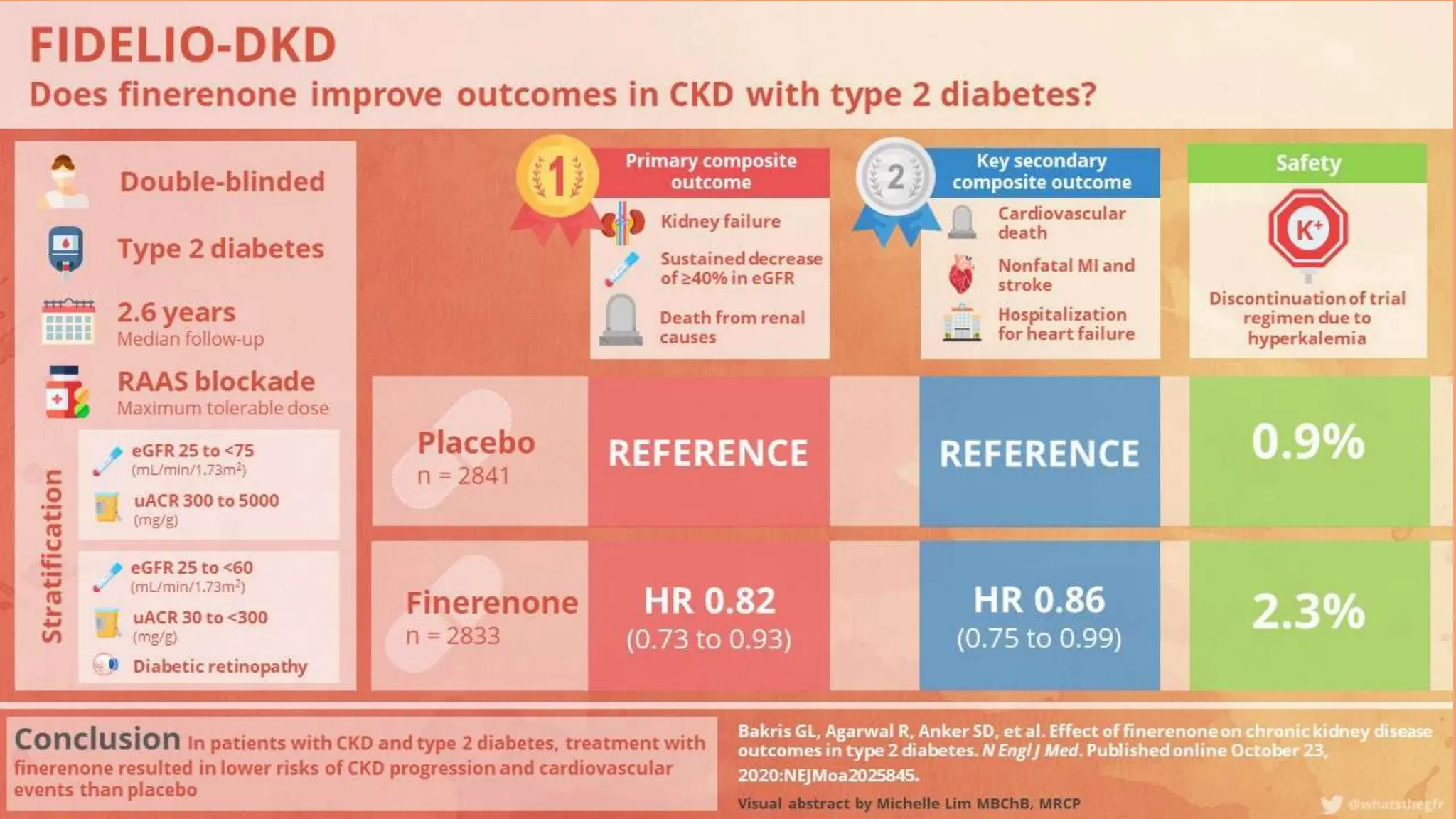
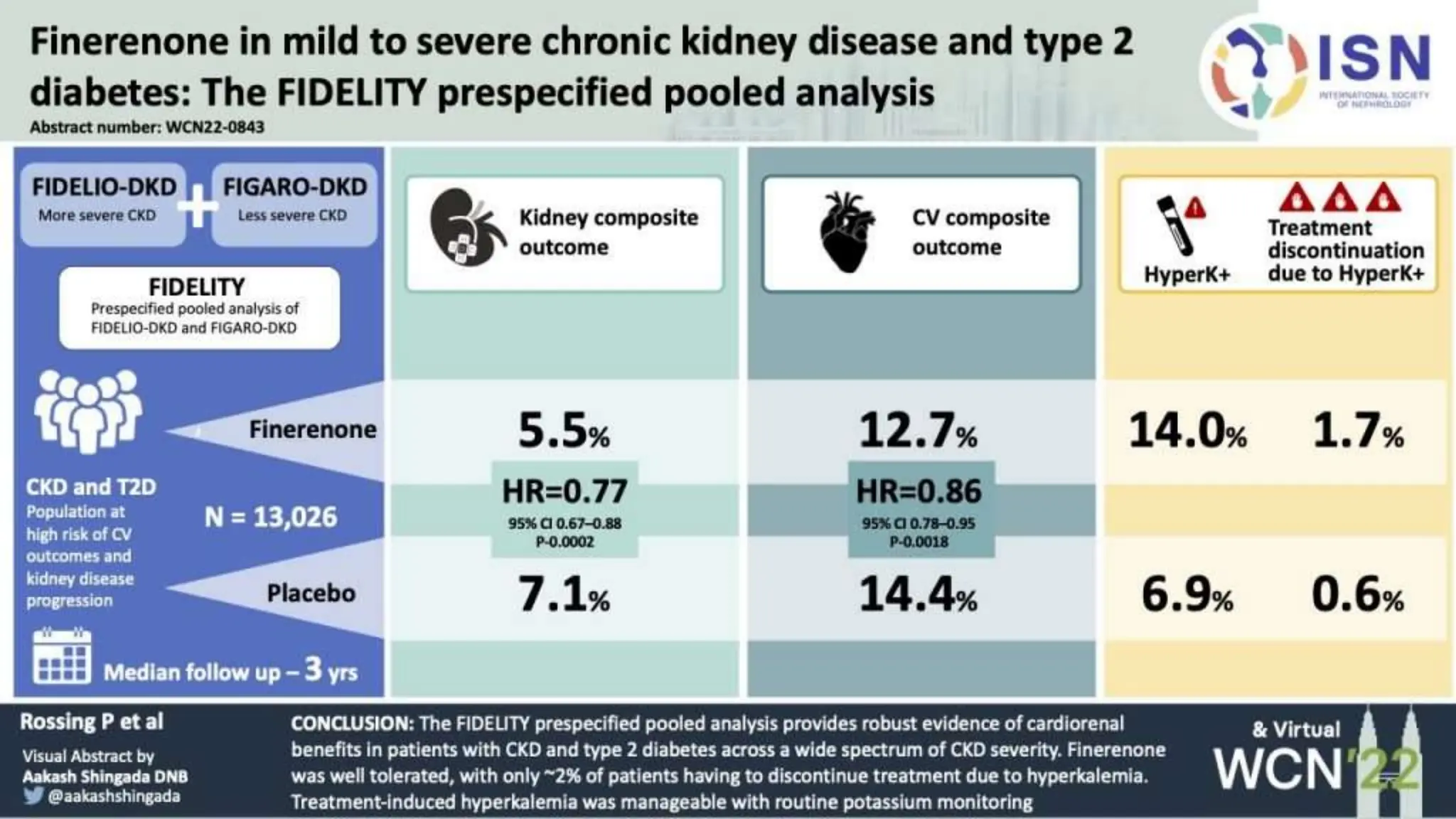
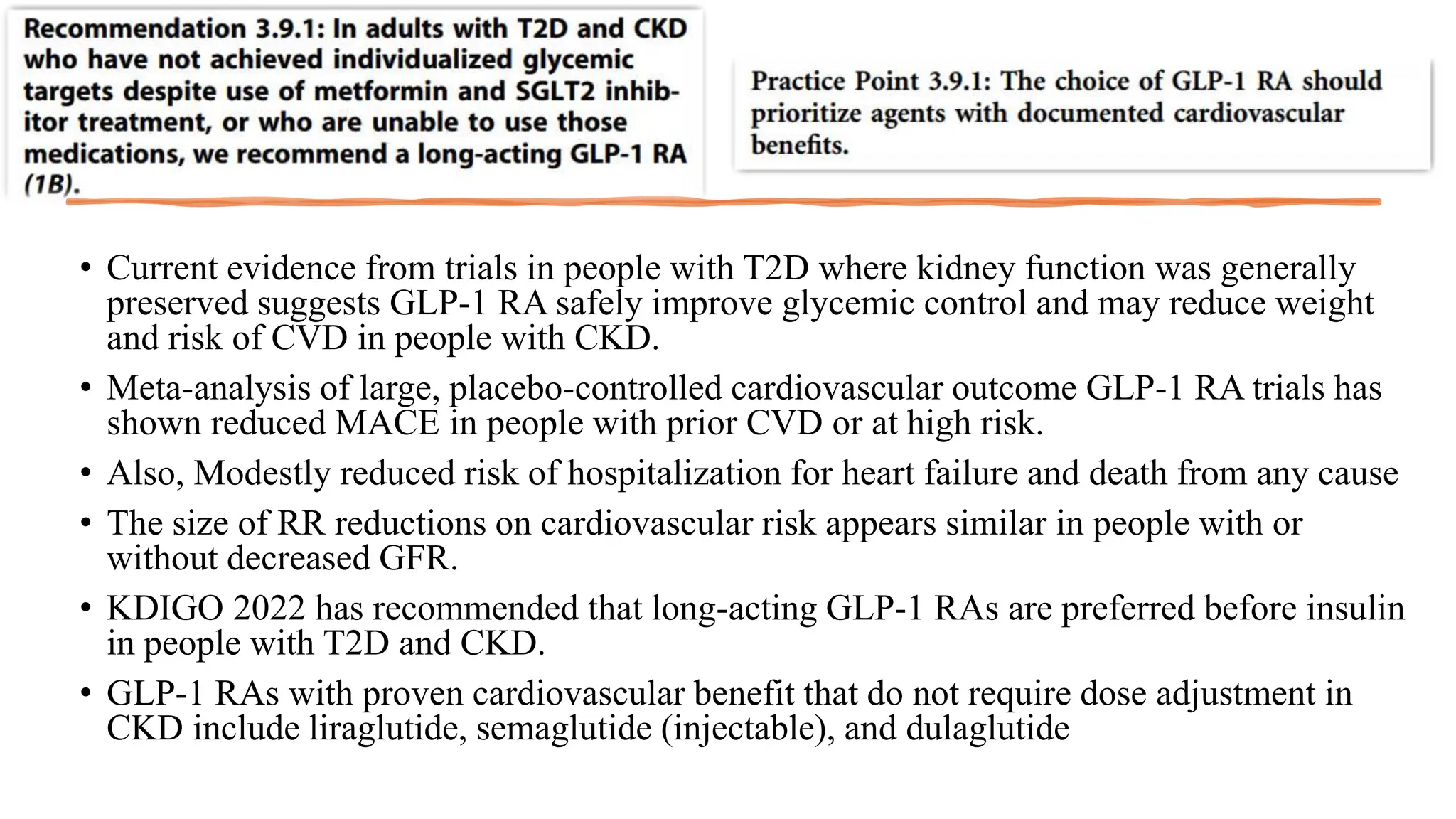
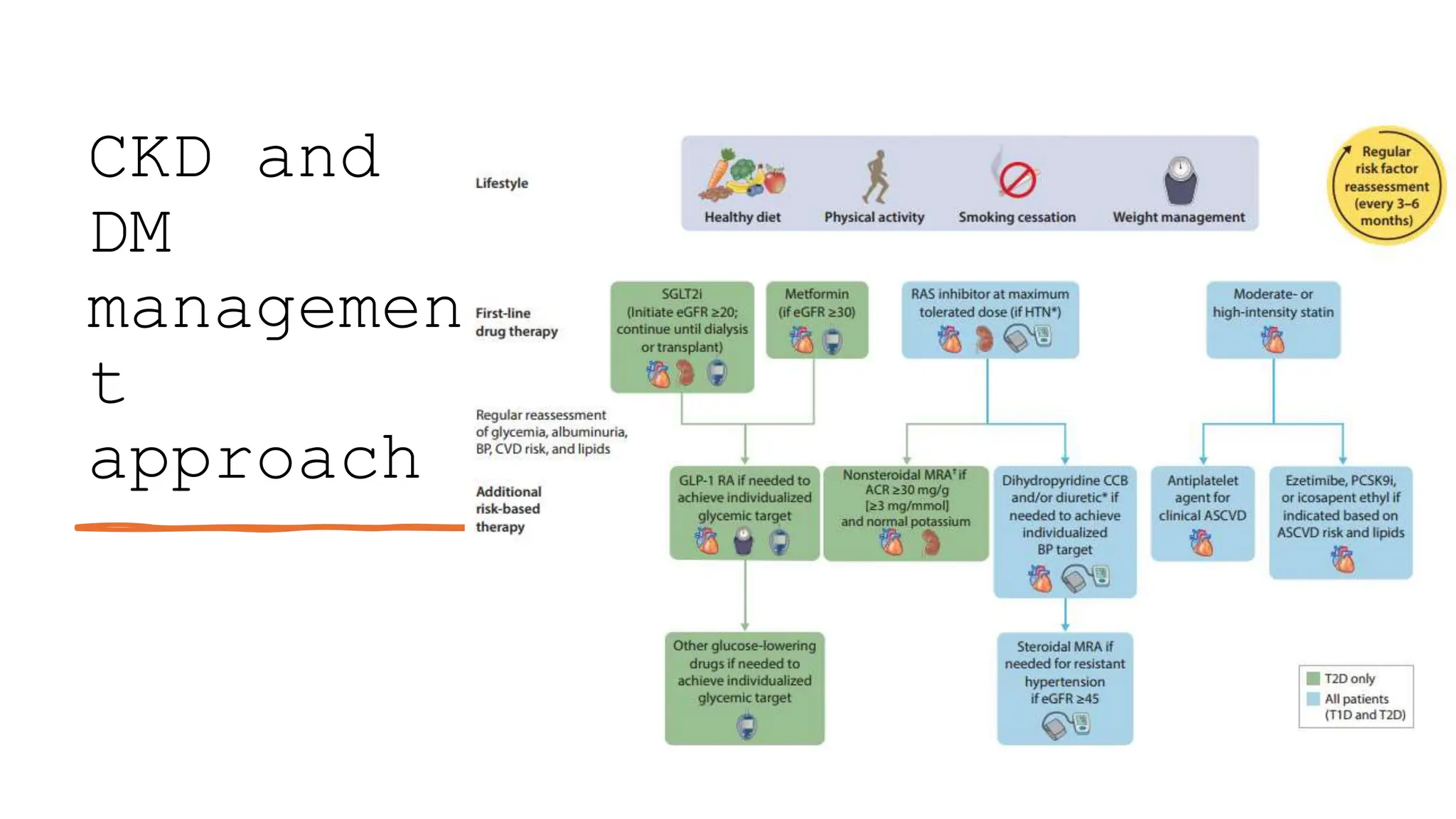
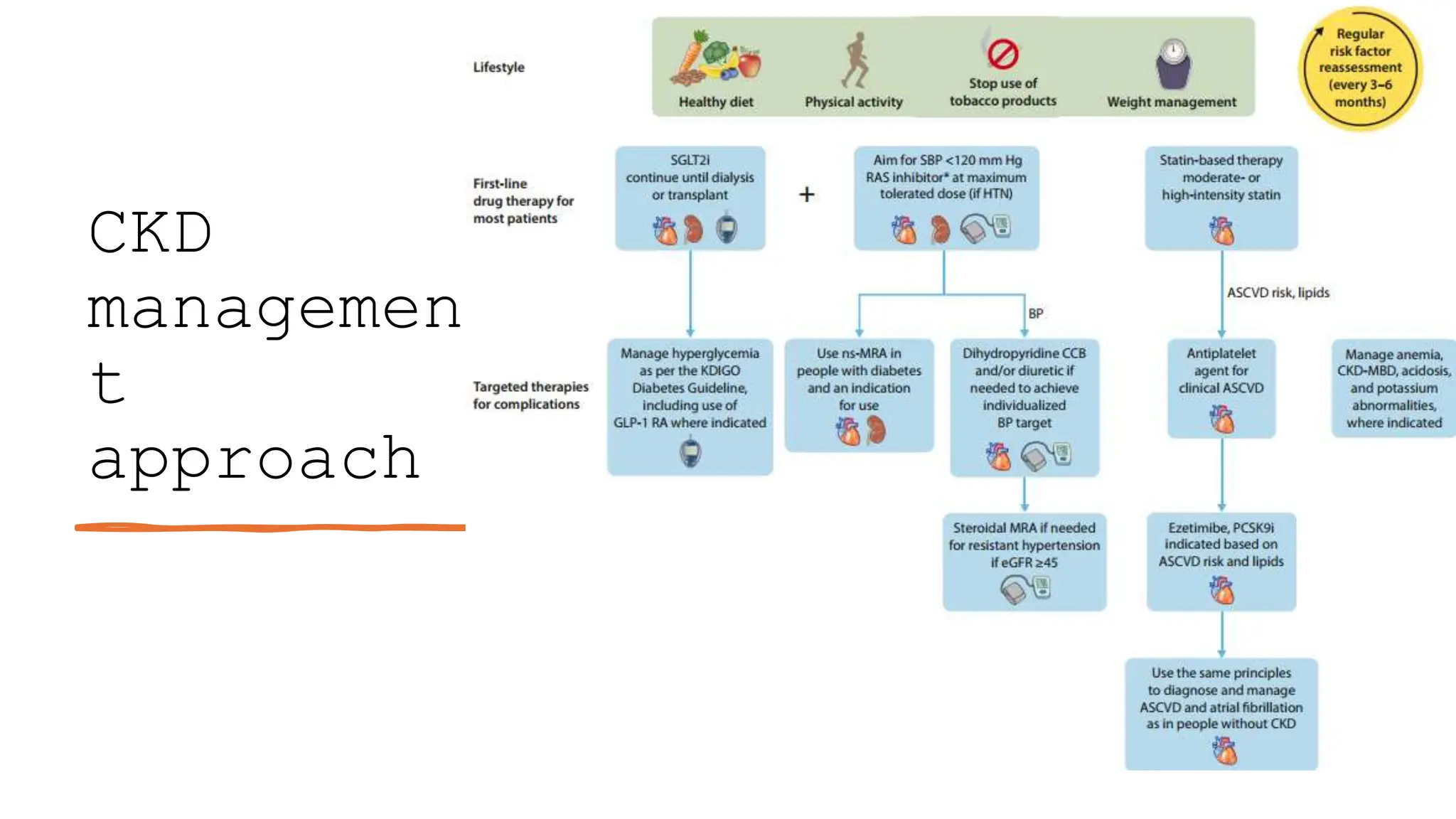
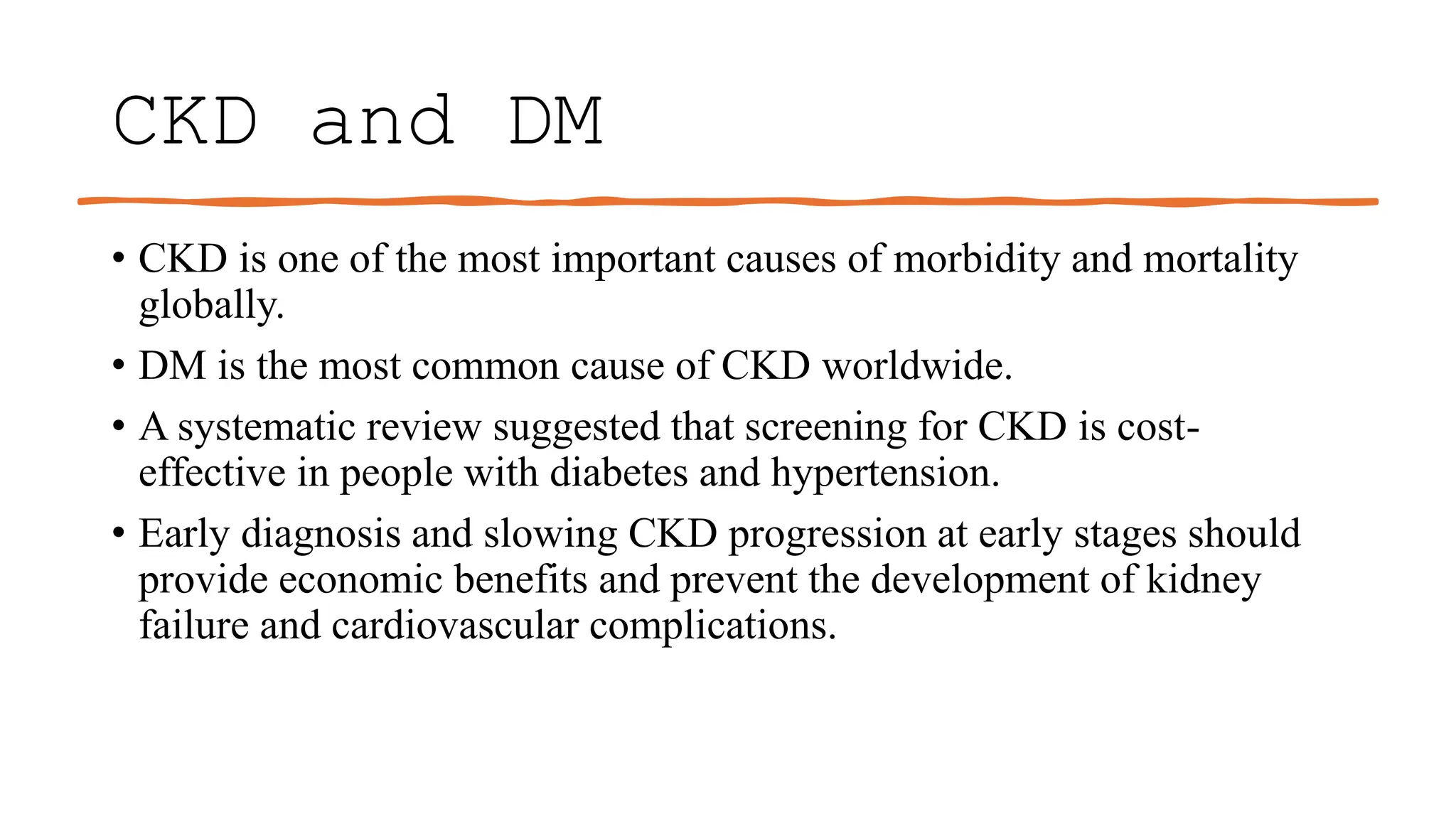
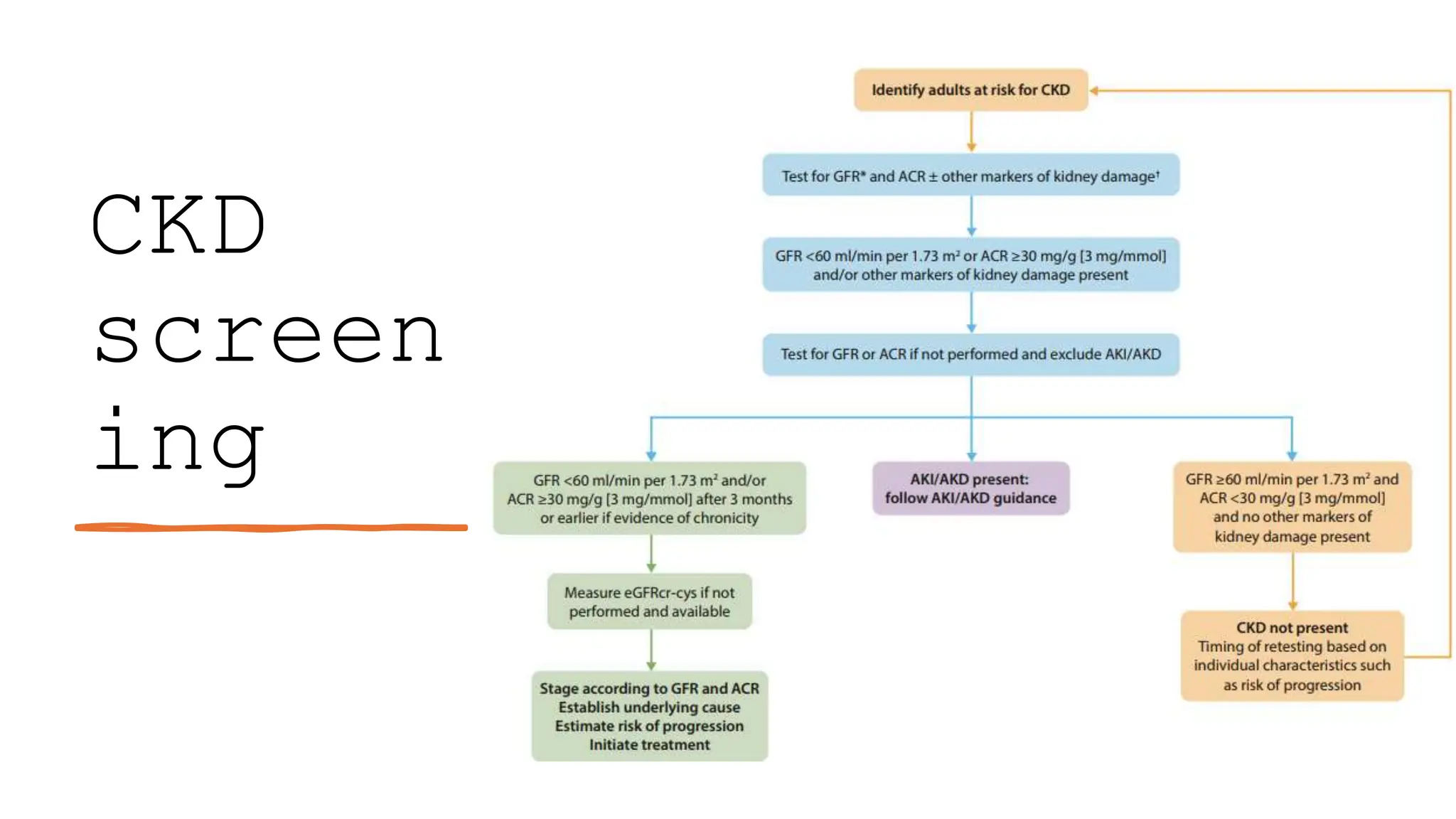
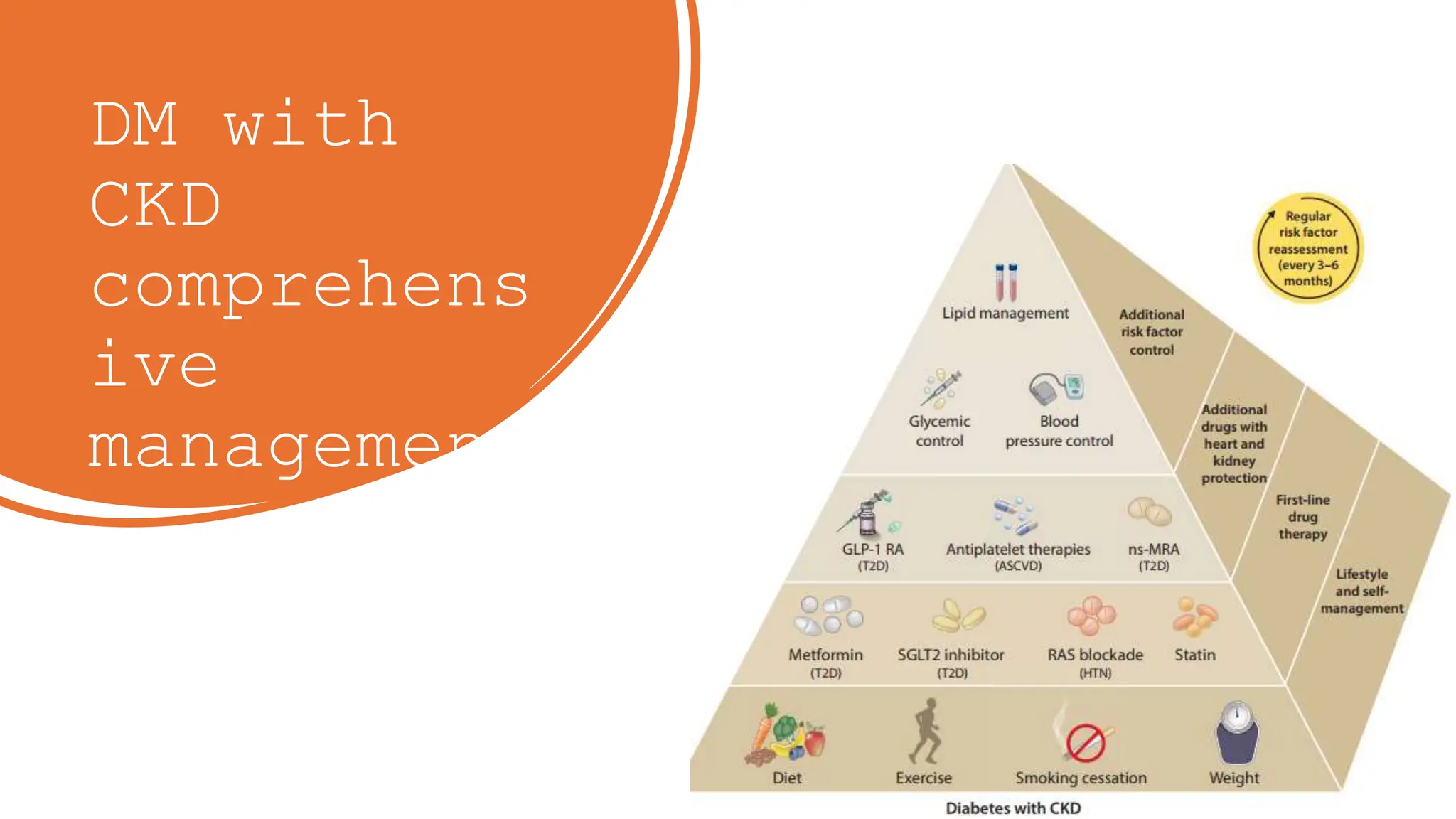
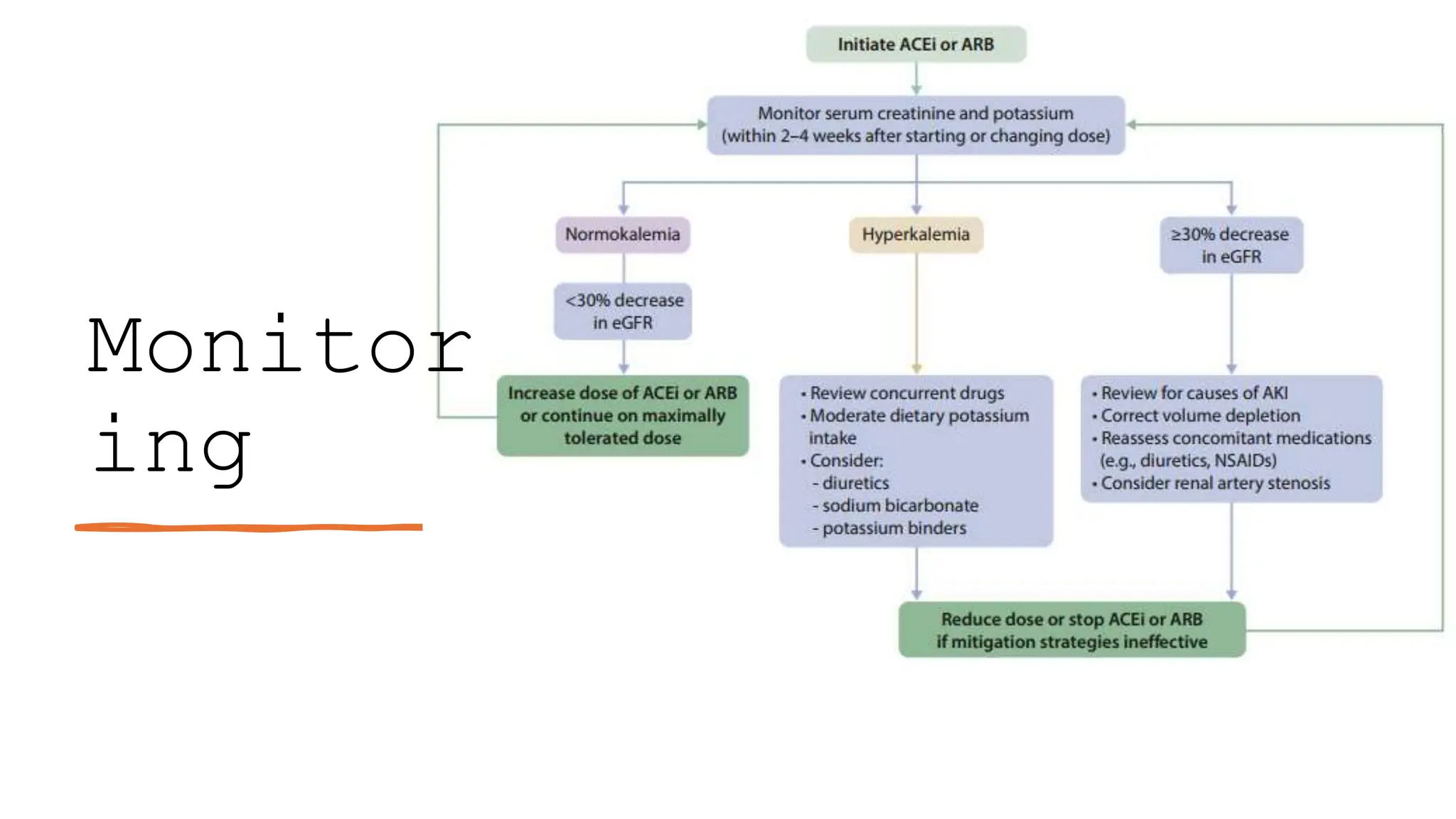
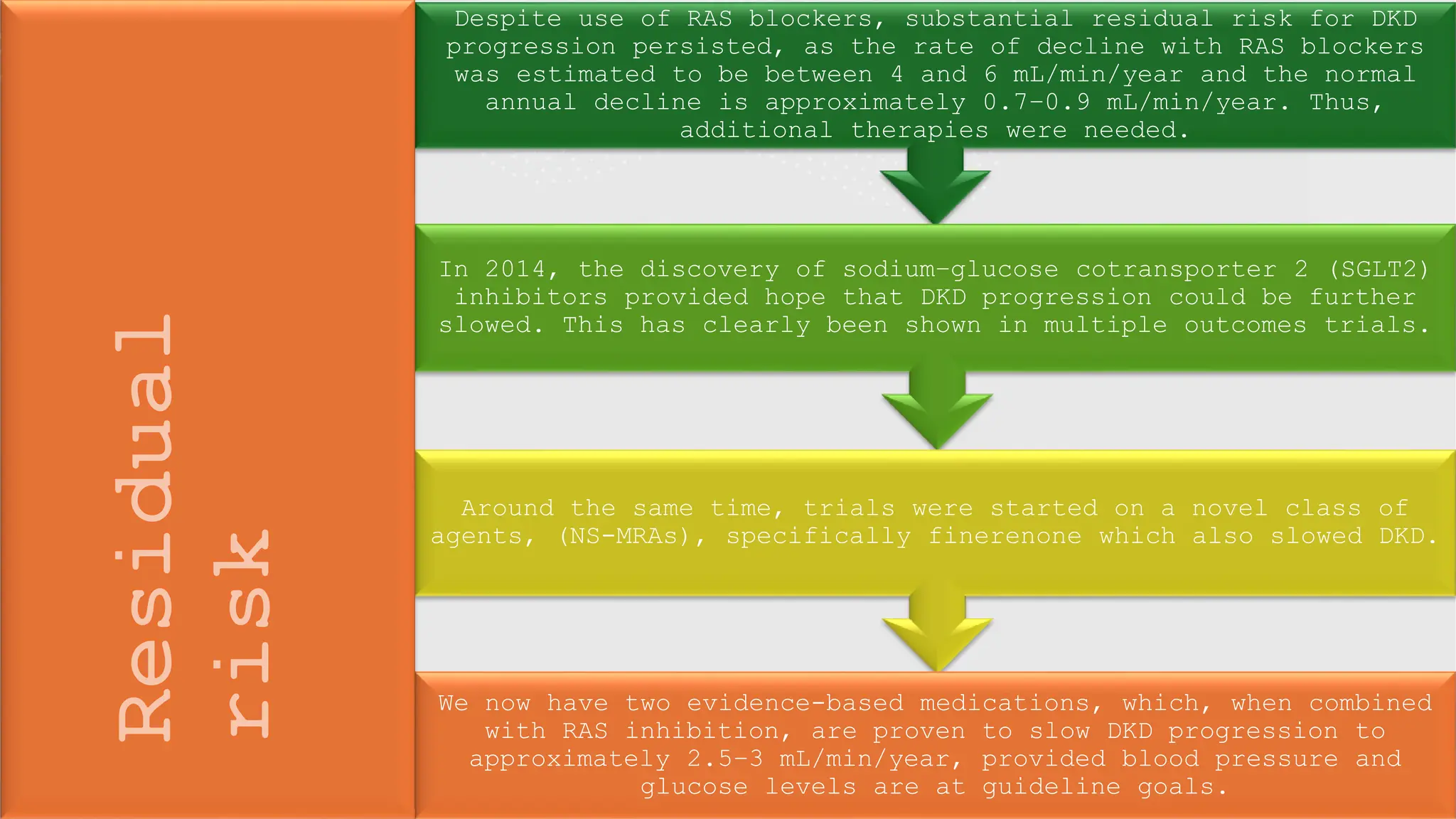
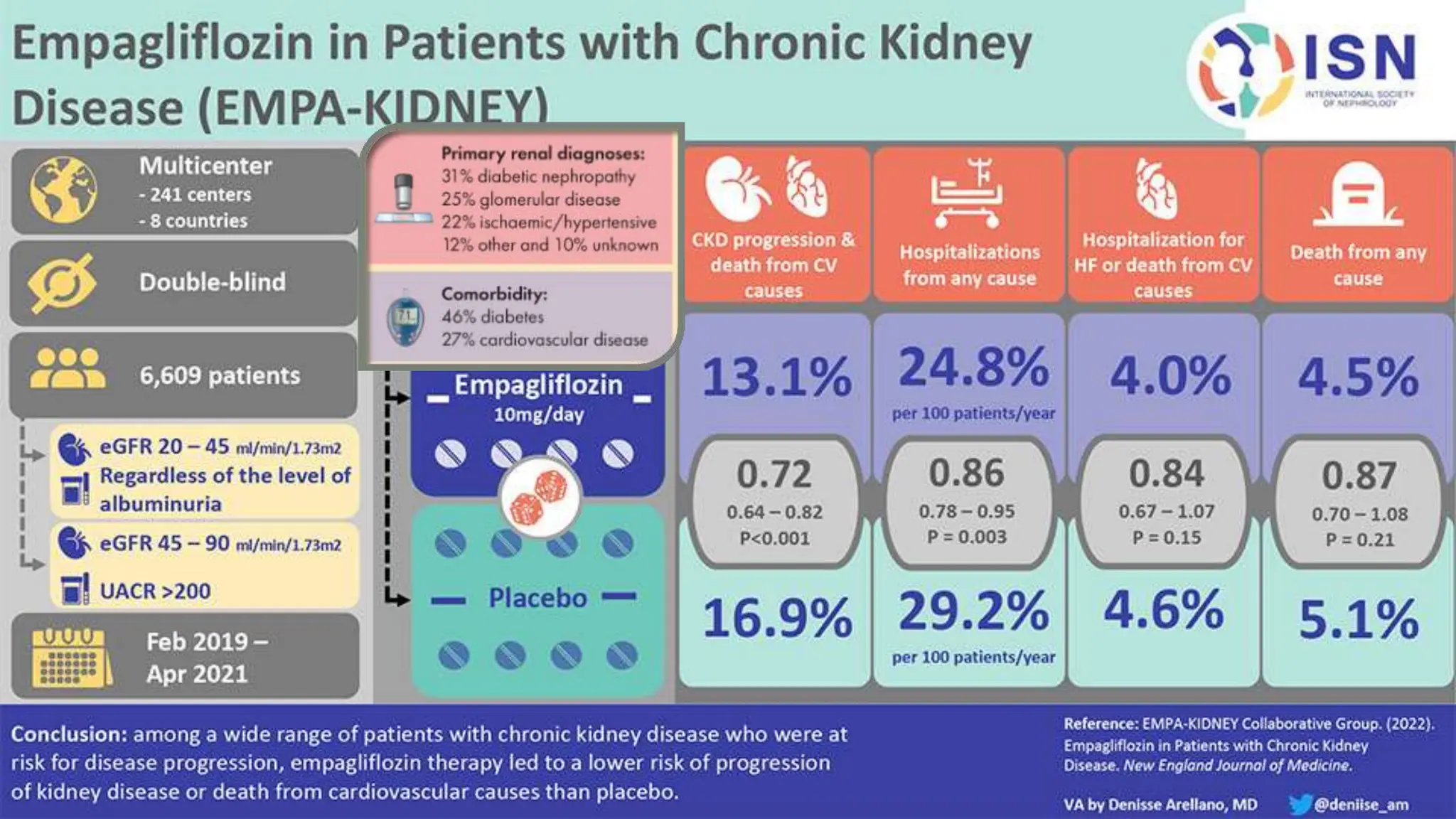
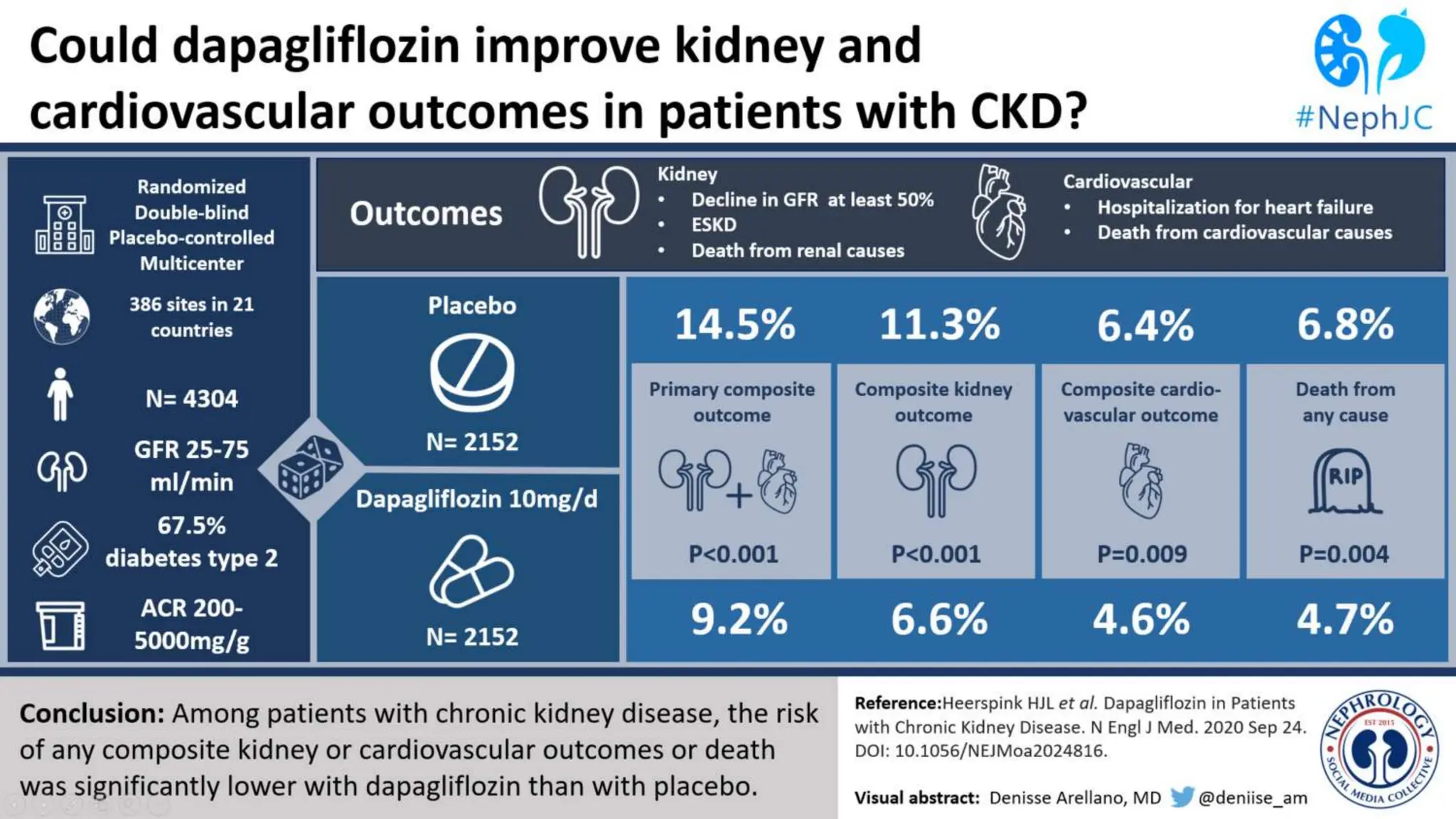
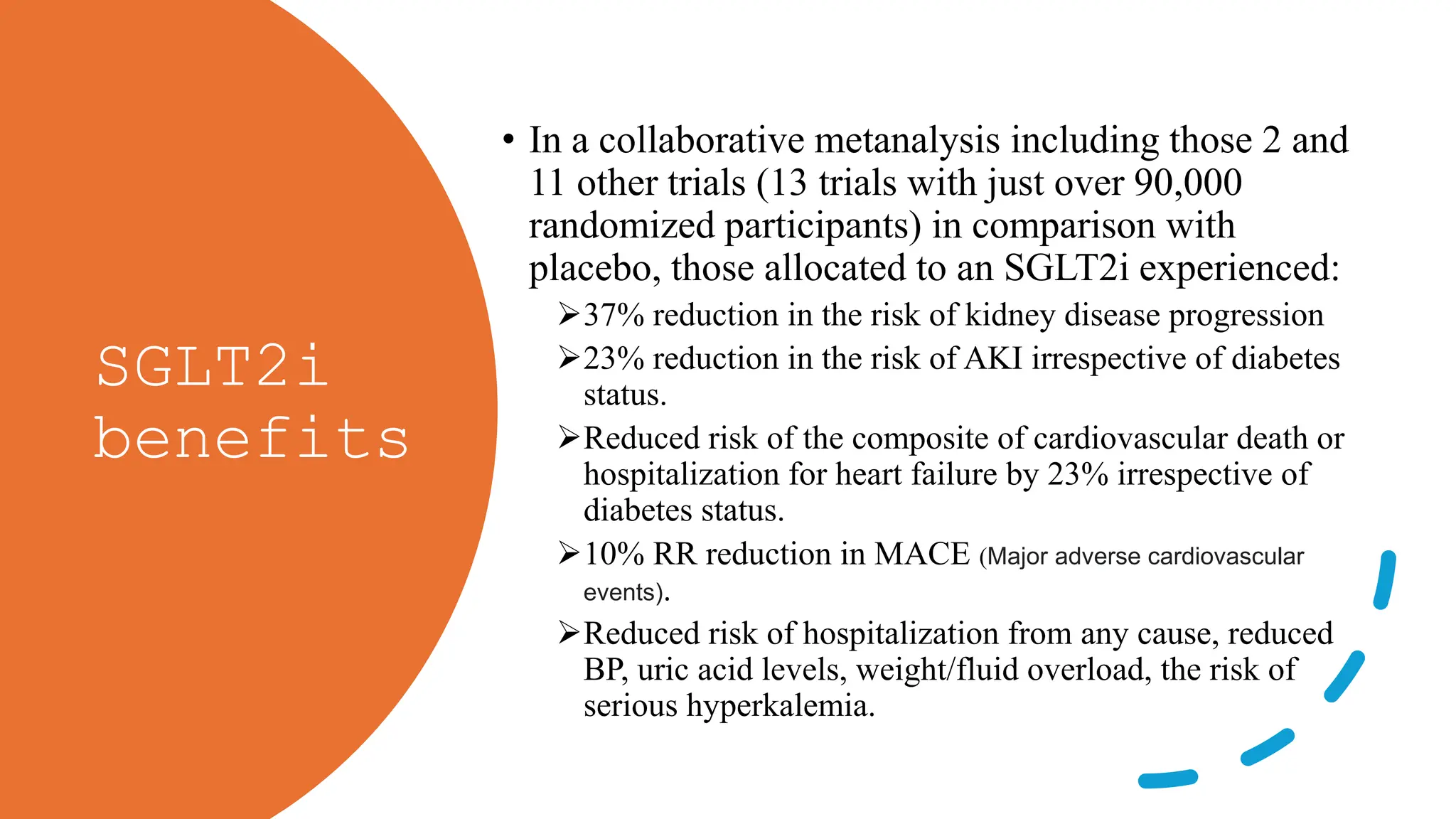
The KDIGO 2024 guidelines focus on improving kidney disease outcomes, particularly chronic kidney disease (CKD) and its relationship with diabetes mellitus (DM). It emphasizes the importance of early screening and management strategies, including SGLT2 inhibitors and non-steroidal mineralocorticoid receptor antagonists, to slow CKD progression and provide cardiovascular benefits. The guidelines highlight the efficacy and safety of various treatments while noting the economic advantages of managing CKD in diabetic patients.









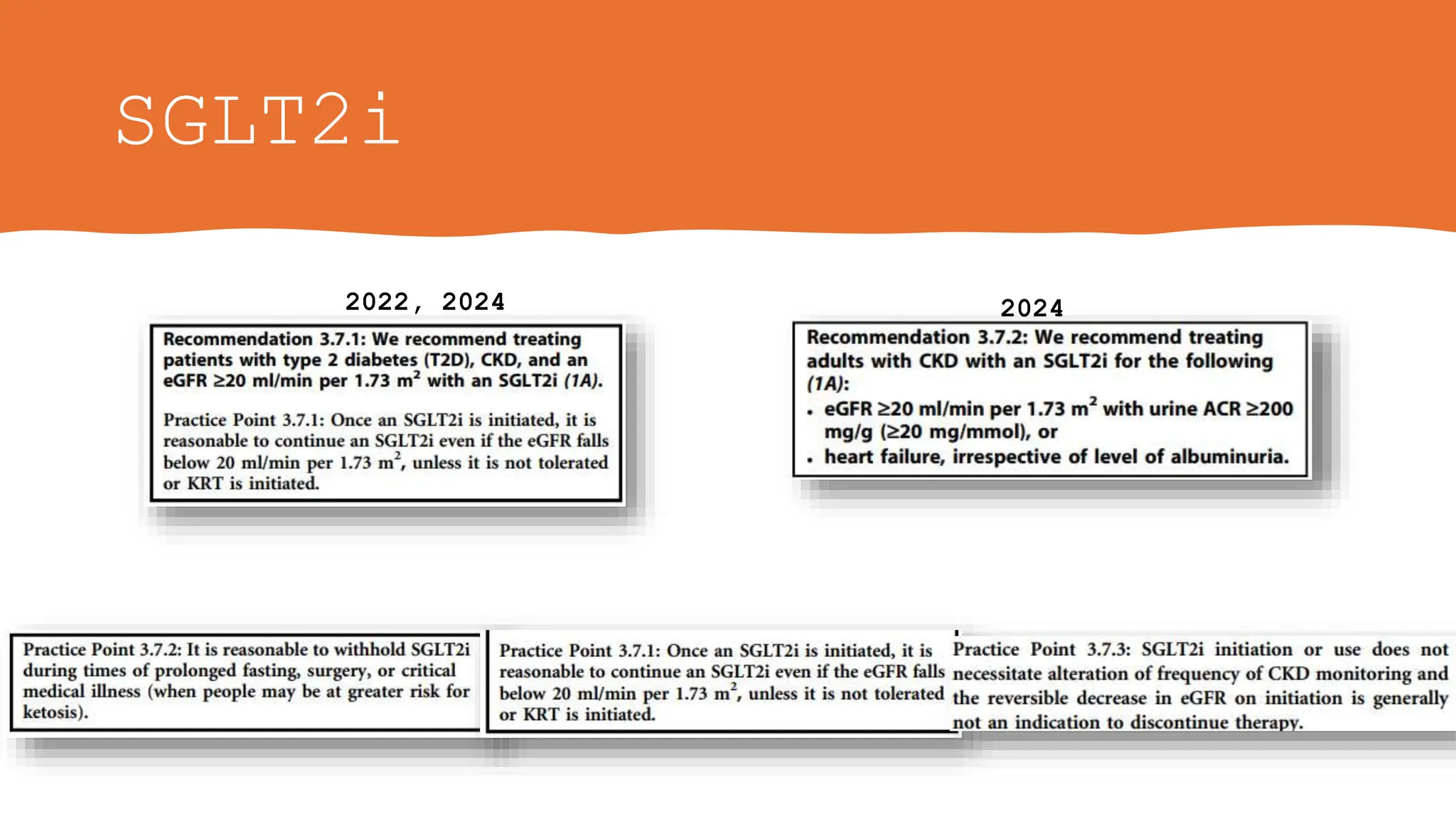
![SGLT2i
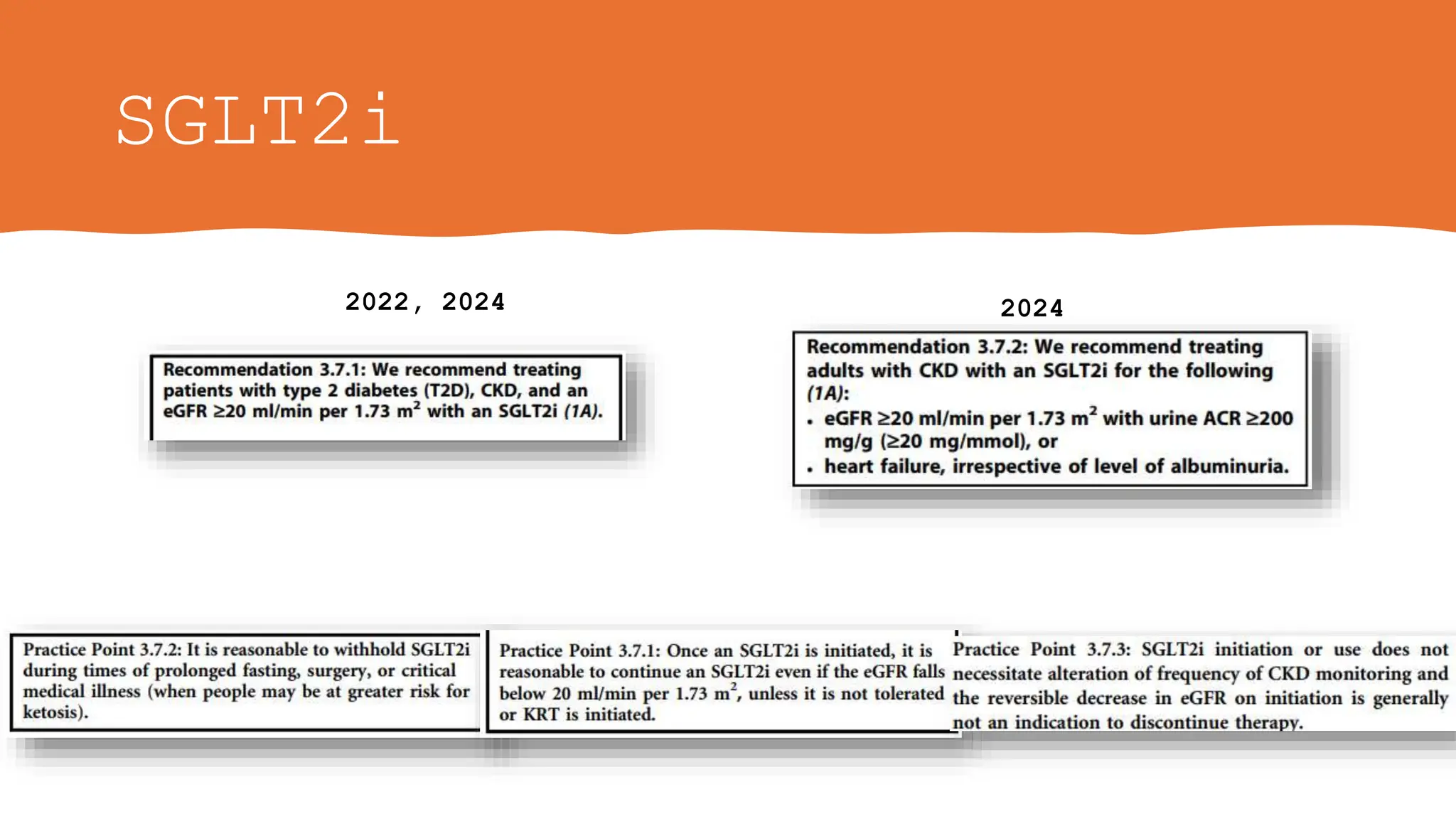
• The Work Group judged that fully informed adults without diabetes and low levels of
albuminuria (urine ACR <200 mg/g [<20 mg/mmol]) who have established CKD and an
eGFR of 20–45 ml/min per 1.73 m2 may be particularly motivated to take SGLT2i for the
benefits identified on rate of decline in GFR
• Evidence for benefits on CKD progression in people without diabetes and with low levels of
albuminuria is limited to eGFR slope analyses in heart failure trials and one CKD trial all with
relatively short follow-up periods. However, extrapolation of these eGFR slope results
suggests that important benefits would arise for such people if treated long term and the
initiation of RRT can be delayed.](https://image.slidesharecdn.com/kdigo2024forendocrinologists-240601195410-97d735da/75/KDIGO-2024-guidelines-for-diabetologists-26-2048.jpg)