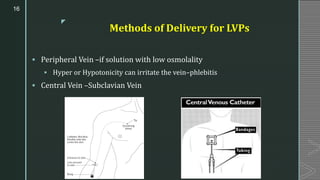
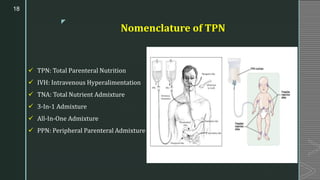
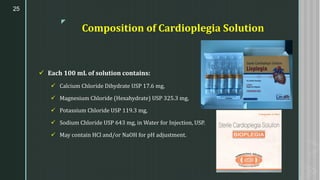
This document discusses large volume parenterals (LVPs), which are intravenous solutions intended for administration of more than 100 mL. It describes the characteristics, containers, labeling requirements, commonly used solutions like sodium chloride, dextrose, Ringer's solution and lactated Ringer's solution. It also discusses types of LVPs including electrolyte, carbohydrate, and nutritional solutions. Large volume parenteral containers can be plastic bags or glass bottles. Total parenteral nutrition solutions, cardioplegia solutions, peritoneal dialysis solutions, and irrigating solutions are also summarized. Formulation considerations for LVPs like drug-excipient compatibility, selection of containers, solubility of active ingredients, and pH are highlighted.