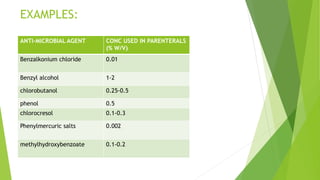
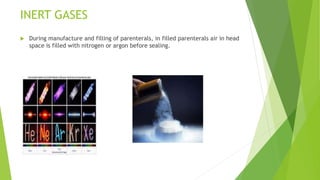
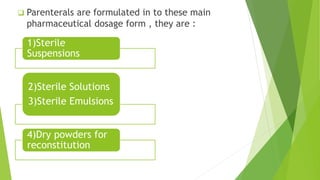
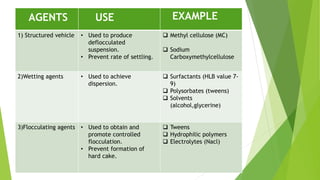
Parenterals are formulated as solutions, suspensions, emulsions, powders, or nano systems. They contain an active ingredient, vehicle, and added substances to maintain stability and sterility. Added substances include solubilizers, antioxidants, chelating agents, antimicrobial preservatives, buffers, tonicity contributors, and protectants. Parenterals are formulated to be sterile and in stable forms like suspensions, solutions, emulsions or powders for reconstitution to facilitate easy administration while maintaining purity and therapeutic activity. They undergo quality testing for leakage, clarity, pyrogenicity and sterility.