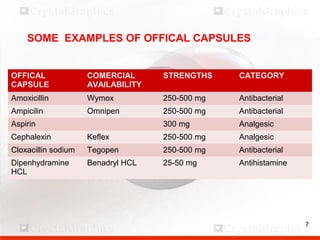
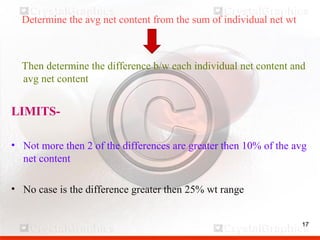
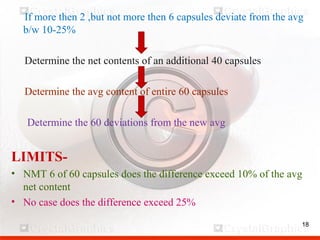
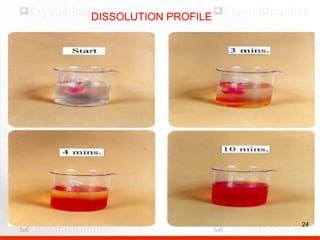
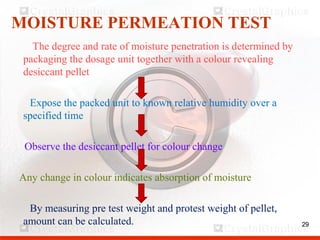
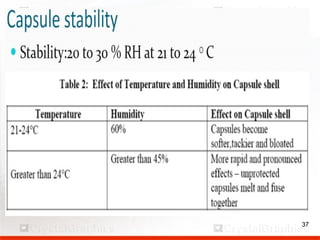
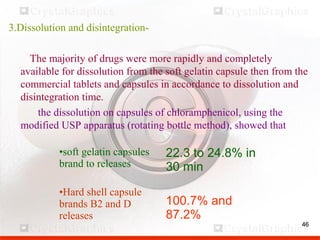
Control including pharmaceutical aspects, physical stability and packing of capsules. Capsules provide advantages such as masking taste and odor, ease of swallowing, and economical production. Quality control tests include physical tests like disintegration, weight variation and chemical tests like dissolution and content uniformity. Capsules are packaged in containers like plastic bottles or blister packs to protect from moisture and ensure stability. Pharmaceutical aspects of capsules include improved dissolution and bioavailability over tablets due to liquid fill formulations, as well as reduced gastric irritation potential.