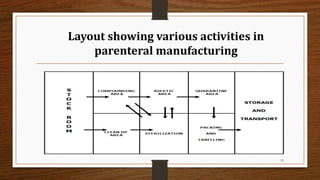
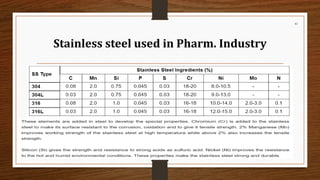
The document provides a comprehensive overview of small volume parenterals (SVPs), including their classification, types of vehicles, and preparation methods. It covers the specifics of single-dose and multiple-dose vials, various vehicle types such as aqueous and non-aqueous options, and the importance of maintaining sterility during manufacturing. Additionally, it discusses the critical aspects of processing, manufacturing areas, and the roles of various additives in ensuring product stability and safety.