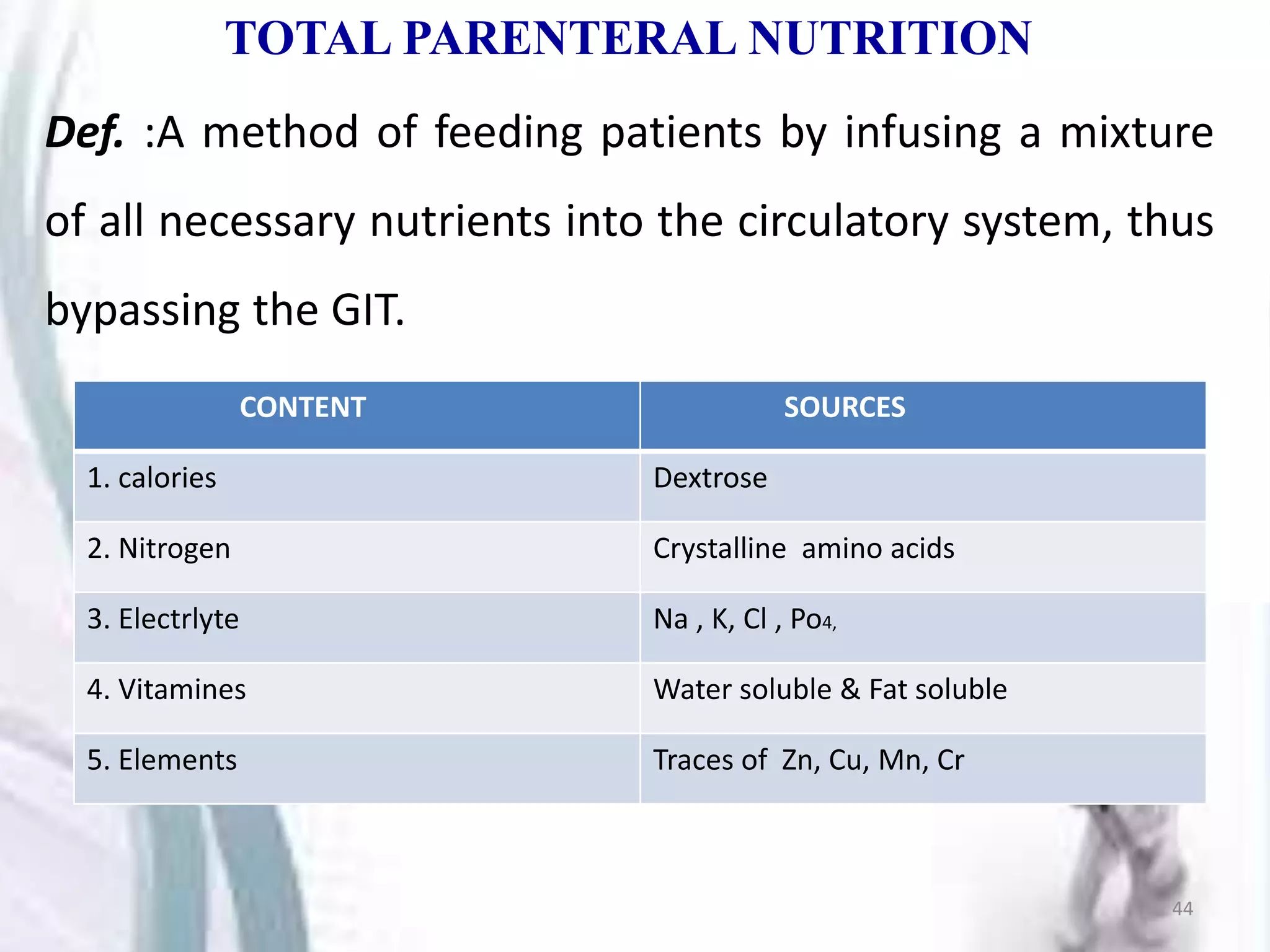
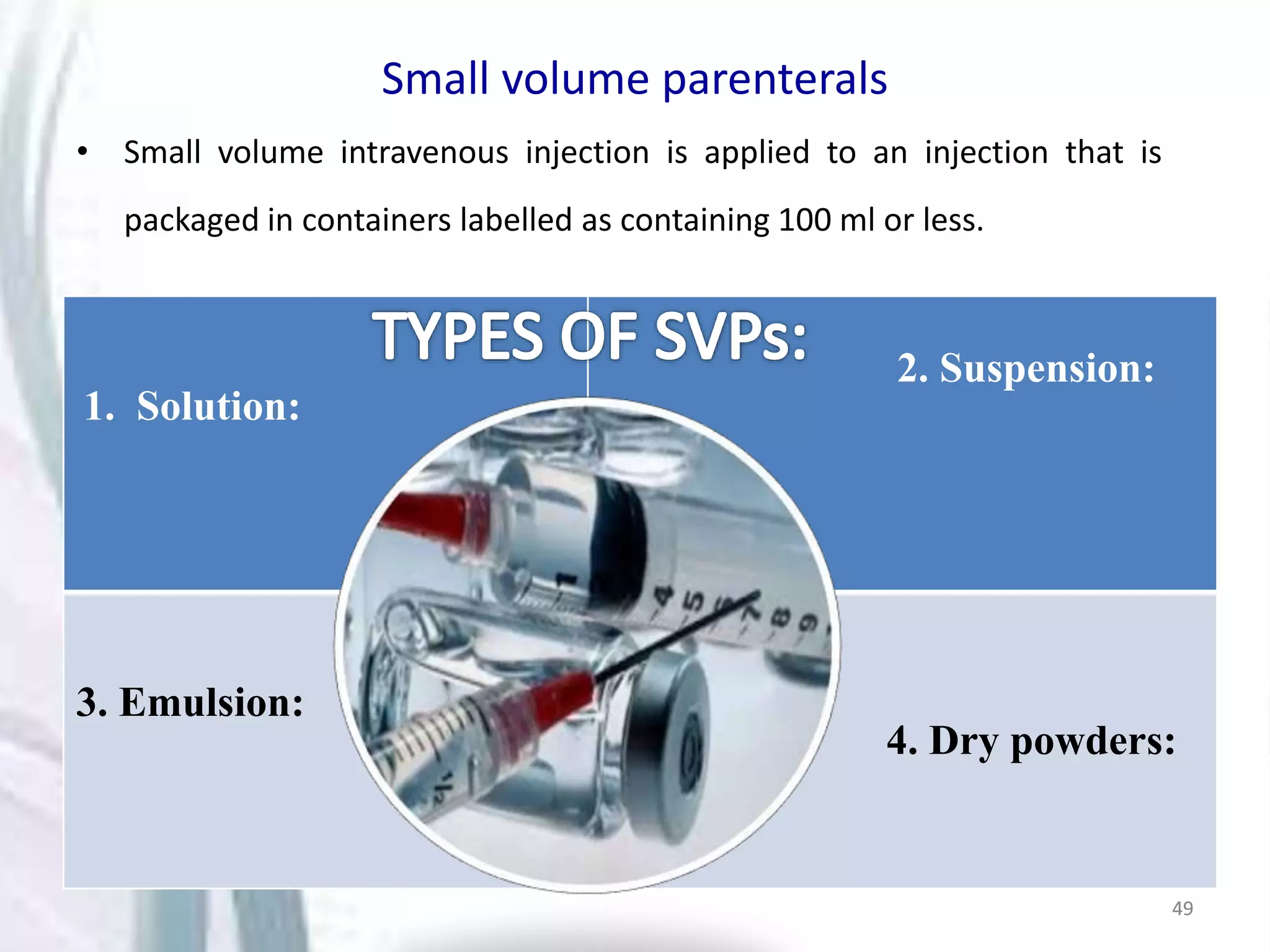
Parenterals are sterile solutions or suspensions of drugs administered through routes other than the gastrointestinal tract. This document discusses various aspects of parenterals including their routes of administration, requirements for stability and sterility, and types such as small volume parenterals and large volume parenterals. It provides details on the production of water for injection and various parenteral vehicles, formulations, and examples.