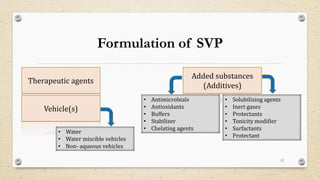
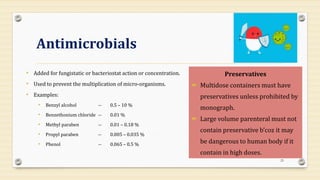
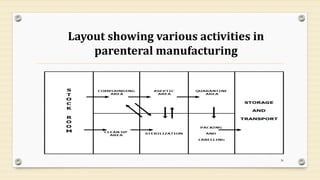
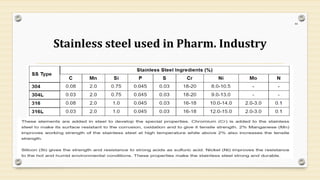
This document provides information about small volume parenterals (SVPs). It defines SVPs as injections packaged in containers of 100ml or less. SVPs can include pharmaceutical products, biological products, and more. They are commonly classified as single dose ampoules, single dose vials, multiple dose vials, and prefilled syringes. The document discusses vehicles, additives, processing, and more regarding the formulation and manufacturing of SVPs.