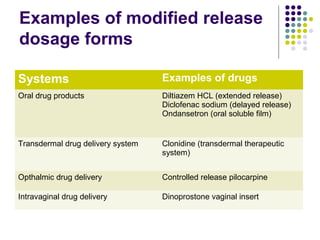
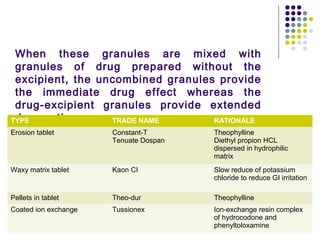
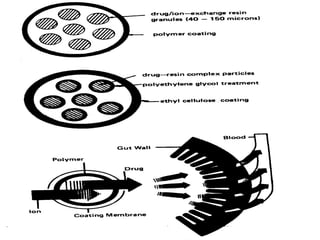
This document discusses modified release drug products, including extended release and delayed release dosage forms. It defines modified release as altering the timing and/or rate of drug release. Extended release aims for twice daily dosing by providing continuous drug levels while delayed release releases the drug at a time other than promptly after administration. The document discusses various extended release drug delivery technologies like coated beads, multilayer tablets, microencapsulation, embedding in eroding matrices, plastic matrices, complexation, and osmotic pumps. It emphasizes the importance of in vitro-in vivo correlations and bioequivalence studies in evaluating these modified release products.






![KINETICS OF EXTENDED
RELEASE DOSAGE FORM
Dtotal = DI + DM
Here, Dtotal = total dose required
DI = initial dose released
DM = sum of maintainence dose
also , DM = k˚r td
so, [ Dtotal = DI + k˚r td ]
However maintainence dose is released after DI
has produced a blood level equal to the
therapeutic drug level.](https://image.slidesharecdn.com/modifiedreleasepresentation-170529012354/85/Modified-release-drug-products-8-320.jpg)


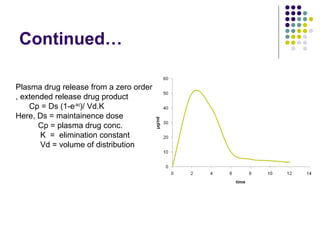




















![2. STEADY STATE PLASMA DRUG
CONCENTRATION
Fluctuation = C∞
max - C∞
min /C∞
av
where C∞
av is equal to [AUC]/T](https://image.slidesharecdn.com/modifiedreleasepresentation-170529012354/85/Modified-release-drug-products-39-320.jpg)



