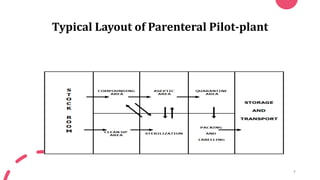
The document discusses pilot plant scale up for sterile veterinary parenteral solutions (SVPS). It defines key terms like plant, pilot plant, and scale up. The objectives and steps of scale up are outlined. The document describes the typical layout and working areas of a parenteral pilot plant, including warehousing, compounding, aseptic, and HEPA filter areas. It discusses evaluating laboratory studies and producing samples at the pilot plant scale. The document also covers different types of parenteral dosage forms that may be tested at a pilot plant, such as solutions, suspensions, emulsions, and dry powders.